Translate this page into:
Snakebite
*Corresponding author: Archana Aher, Associate Professor, Department of General Medicine, Government Medical College and Hospital, Nagpur, Maharashtra, India. drarchanaaher@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Asudani D, Aher A. Snakebite. Vidarbha J Intern Med 2022;32:35-41.
Abstract
Snakebite is a prevalent cause of morbidity and mortality in rural India. There is a great unawareness among the general public about this important occupational hazard and timely intervention like anti-snake venom. Furthermore, there is a confusion among primary health centre workers about the management due to various Western guidelines which are difficult to follow in the Indian setting. Knowledge about its prevention, avoiding harmful first aid measures, and having proper guidelines for its management can help in timely proper intervention and saving lives. Hence, here, we present a short review on types of snakes, clinical features, guidelines of management (based on Indian protocols), and its prevention.
Keywords
Snake Bite
Indian protocols
Western guidelines
INTRODUCTION
Snakebite is an acute life threatening but preventable public health hazard often faced by rural populations in tropical and subtropical countries with heavy rainfall and humid climate. There are around 2000 species of snakes in the world, about 300 species are found in India out of which 60 are venomous.[1] Even though snakebite is a century old life-threatening condition, it was included in the list of neglected tropical diseases by the World Health Organization (WHO) in the year 2009.[2,3] Lack of knowledge about occupational hazards, not reaching hospitals on time, and harmful practices are the main causes of morbidity and mortality due to snakebite in India.
EPIDEMIOLOGY
Around 81,000–138,000 people die each year from snakebites worldwide.[4] In 2019, the WHO launched a strategy to halve the number of death and cases of serious disability by 2030, compared to 2015. Non-fatal bites may be as high as 1.4 million/year, with approximately 1.2 million snakebite deaths in India from 2000 to 2019.[5,6]
Incidences being more common in males (59%), at ages 30–69 years (57%), from June to September (48%), and occurring outdoors (64%). Of the treated, nearly one-third were first attended in the medical centre after 6 h. Deaths mostly occurred at home in the rural areas.[6]
SNAKES IN INDIA
Some of the common poisonous snakes in the Central India are common cobra (Naja naja), Russell’s viper (Daboia russelii), Indian krait/common krait (Bungarus caeruleus), saw-scaled viper (Echis carinatus), bamboo pit viper/Indian green pit viper (Trimeresurus gramineus), the Malabar pit viper/rock viper (Trimeresurus malabaricus) and green vine snake (Ahaetulla nasuta).[7] [Figures 1-6] shows common snakes found in Maharashtra region, along with their identifying properties. Anti-snake venom (ASV) in India is available against only ‘big four’ that is, common cobra (N. naja), common krait (B. caeruleus), Russell’s viper (D. russelii), and saw-scaled viper (E. carinatus).[8] Due to various differences in the composition of venom of different species of snakes, Sunagar et al. suggested that venoms of locally, medically relevant snakes must be used to produce antivenom that will work more efficiently in that region.[1,8] Matthew Lewis, founder of Bay Area Startup Ophirex, identified Varespladib, a small molecule being very potent against a virulent component of venom called sPLA2, found in abundance in many of the world’s venomous snakes.[1]

- Indian cobra. Huge nostrils, black eyes with rounded pupil, and a spectacle symbol is evident on stretching its hood.

- Russell viper. Even scaled, slightly wide head than its neck with black eyes.

- Common krait. Coarse scaled snake with big eyes, a broader head than the neck, and a thick body.

- Saw-scaled viper. Trilateral head, which is extensive than its neck.

- Indian green pit viper.

- The Malabar pit viper. Slim green body and arrow-like head.
CLINICAL PRESENTATION
Various factors on which presentation of snakebite victim depend on species, amount of venom injected, season, site, area covered or uncovered, dry or incomplete bite, number of bites, venom injection in the vessel, the weight of the victim, and time elapsed between the bite and administration of ASV. Venom concentration and constitution depend on environmental conditions along with the snake’s maturity and darkness.[3] Snake venoms are complex mixtures of enzymes, polypeptides, glycoproteins, and some deleterious components being proteolytic enzymes that cause local tissue necrosis, affect the coagulation pathway at various steps, and impair organ function.[9] Toxic effect on the cellular level and symptoms resulting from different types of Snake venoms are shown in [Figures 7 and 8].
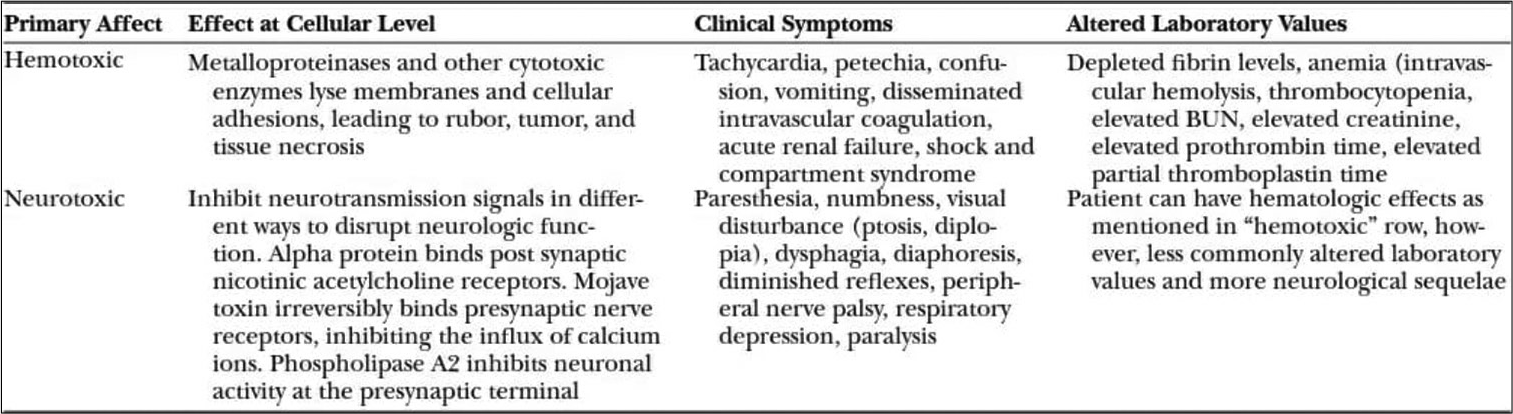
- Toxic effect on the cellular level and symptoms resulting from it.
- General approach according to the different presentations.
Common krait bite may not be painful and the local symptoms are barely discernible but neuroparalysis may be associated with abdominal pain and hypokalaemia. These neurotoxins act presynaptically and prevent the release of acetylcholine at the neuromuscular junctions.
Russell viper venom can cause local and haemotoxic manifestations with distinctive blistering of the affected limb. Haematuria, renal failure, and oedema typically complicate Russell’s viper envenomation.
Saw-scaled viper can cause ecchymosis, haematological complications, local pain, and oedema.
Common sea snake venom possesses potent post-synaptic neurotoxic activity and even though bites may be inconspicuous, painless, free of oedema, neurological complications, oliguria, and hyperkalaemic cardiac arrest are seen in severe envenomation.
Cause of death – The cause of death in a snakebite case can be respiratory failure due to neuroparalysis, haemodynamic instability (such as bleeding and hypotension), acute renal failure, disseminated intravascular coagulation, and multiorgan failure.
FIRST AID MEASURES
Recommended first aid
Reassurance, immobilisation, especially the bitten limb (helps in delaying systemic absorption of venom), and accelerated transport to medical care are some of the important first aid measures. Ideally, the patient should lie in the recovery position (prone, on the left side) protecting his/her airway to minimise the risk of aspiration of vomitus.[10] The pressure immobilisation technique is the only evidence-based technique that prevents the systemic spread of venom.[11] If swelling is progressive, the affected extremity can be elevated as long as no systemic symptoms present.[12]
Things to avoid[13-16]
The tourniquet should not be applied
The bite site should not be washed with soap or any other solution
Do not make cuts or incisions on or near the bitten area
Avoid any kind of potentially harmful herbal or folk remedy
Avoid attempts to suck out the venom with your mouth
Avoid giving drink, alcohol, or other drugs to the victim
Do not attempt to capture, handle or kill the snake.
ACUTE HOSPITAL MANAGEMENT
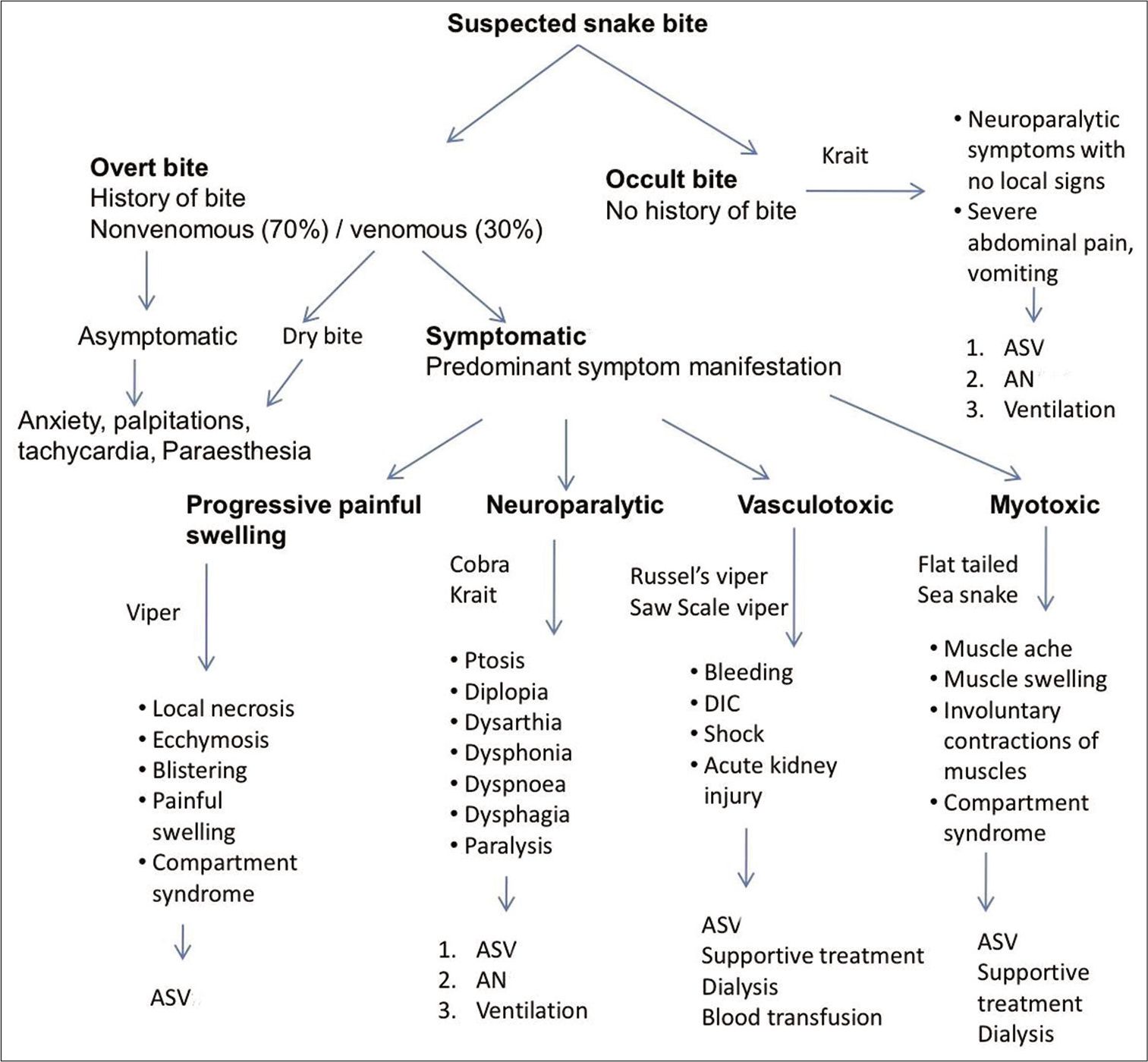
A General Approach on arrival to the medical facility is given in [Figure 9].[9]

- General approach on arrival to the medical facility.
Medical management of envenomed patients in dispensary or hospital
A full patient workup, along with irrigation and careful inspection of the wound, should be done.[17-19] An identified haematological envenomation with no signs of secondary complications should be monitored for a minimum of 12 h to ensure no development or progression of symptoms.[20] If bitten by a neurotoxic snake, a minimum observation period of 24 h is required with specific neurological monitoring to ensure no further progression of symptoms [Figure 10].[17,21]
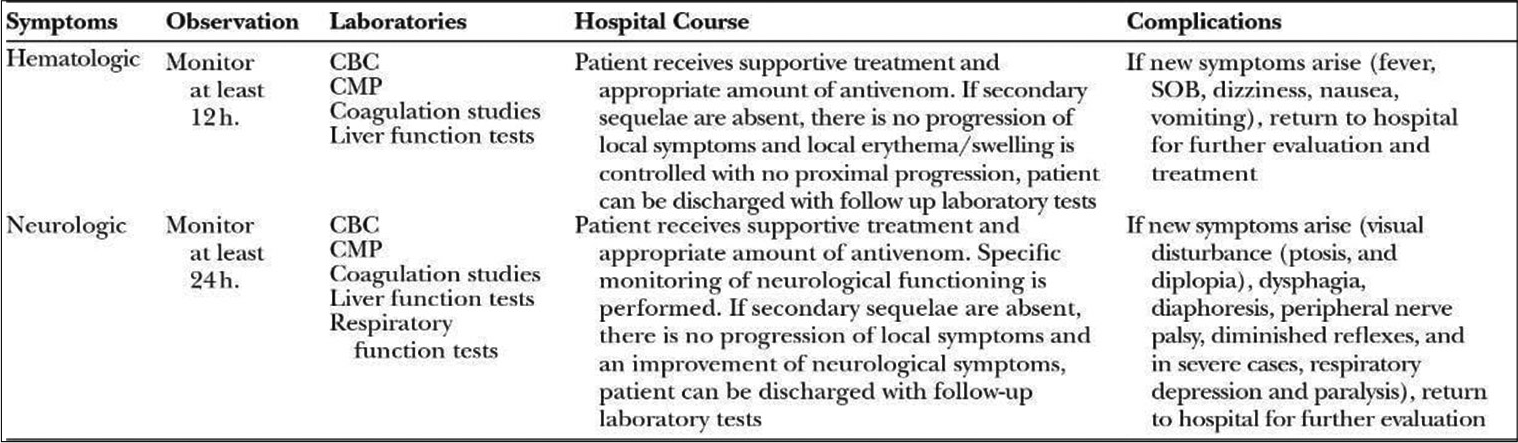
- Monitoring parameters.
20 min whole-blood clotting test
The ordinary glass should be used. Normal control blood can be used for comparison. For evolving venom-induced DIC, the test should be repeated 6 hourly. ASV treatment should be initiated urgently if there is other evidence of haemostatic disturbances (e.g., spontaneous systemic bleeding distant from the bite site).
ASV
ASV is the only specific treatment available against snakebite. It may also be useful in delayed presentations till 2–3 weeks. At present, ASV available in India is made from purified IgG antibodies after injecting venom into the animal host. Early intervention within 4 h and a higher dose of antivenom offer more favourable patient outcomes.[22-24] ASV is ineffective against hump-nosed pit viper (Hypnale hypnale), and questionable against Sochurek’s saw-scaled viper (Echis carinatus sochureki) bit found in Rajasthan. ASV is available in liquid and lyophilised forms with a shelf life of 2 and 5 years, respectively.
Indications for ASV
If a patient of proved or suspected snakebite develops, the following signs and/or symptoms are as follows: [2,4,19,25-28]
Haemostatic abnormalities: Coagulopathy – WBCT> 20 min, INR >1.2 and thrombocytopenia <1.0 lakh per microlitre of blood or spontaneous systemic bleeding distant from the bite site
Neurotoxic signs
Cardiovascular instability
Acute kidney injury
Haemoglobinuria/myoglobinuria
Local swelling involves more than half of the bitten limb (in the absence of a tourniquet) within 48 h of the bite. Swelling after bites on the digits (toes and especially fingers) or a rapidly extending swelling
Development of an enlarged tender lymph node draining the bitten limb.
Contraindications and Prophylaxis of high-risk patients
No absolute contraindication[28]
Patients who have reacted to horse (equine) or sheep (ovine) serum in the past (e.g., after treatment with equine anti-tetanus serum, equine anti-rabies serum, or equine or ovine antivenom) and those with a strong history of atopic diseases (especially severe asthma) are at high risk of severe reactions. In such patients, ASV should only be given if they have signs of systemic envenoming. In a large trial conducted in Sri Lanka, compared with placebo, adrenaline significantly reduced severe reactions to antivenom, supporting the routine use of prophylactic adrenaline in the dose of 0.25 ml of 0.1% solution (0.25 mg) by subcutaneous injection. This is recommended before antivenom treatment, except in old patients with suspected or known cardiovascular disease.
ASV test dose
A test dose is not recommended as no benefit is shown in predicting anaphylactic reaction or late serum sickness.
Administration of ASV
Two methods are recommended:
Intravenous ‘push’ injection
Slow intravenous injection (not more than 2 ml/min). This method has the advantage of being economical and one healthcare professional is beside the patient while administering and can detect early reactions.
Intravenous infusion
Reconstituted freeze-dried or neat liquid antivenom is diluted in about 250 ml of isotonic saline or 5% dextrose in the case of an adult patient and is infused at a constant rate over 30–60 min.
ASV must NEVER be given by the IM route because of poor bioavailability by this route. Furthermore, local injection at the bite site is extremely painful and may increase intracompartmental pressure. All aseptic precautions should be taken to prevent any pyrogenic reactions to ASV.
Dose of ASV
Dose of ASV for neuroparalytic snakebite
Following dosages are advised by indian guidelines, which depends on the type of presentation.[10] ASV 10 vials as an infusion over 30 min can be repeated with the second dose of 10 vials after 1 h if no improvement within the1st h.
Dose of ASV for vasculotoxic snakebite
Two regimens are advised; with a low dose, infusion therapy is equally effective as high dose intermittent bolus therapy and also economical.
Low-Dose infusion therapy – 10 vials for Russell’s viper or six vials for saw-scaled viper as an infusion over 30 min followed by two vials every 6 h as an infusion in 100 ml of normal saline. Continue till clotting time normalises or for 3 days whichever is earlier OR
High-dose intermittent bolus therapy – 10 vials of polyvalent ASV over 30 min as an infusion, followed by six vials 6 hourly as bolus therapy till normalisation of clotting time and/or local swelling subsides
Available ASV is not effective against sea snakebite or pit viper bite.
For neuroparalytic snakebite
‘Atropine neostigmine (AN)’ trial – Atropine 0.6 mg followed by neostigmine (1.5 mg) IV stat and should be repeated with a dose of neostigmine 0.5 mg with atropine every 30 min for five doses. Thereafter, tapering doses at 1 h, 2 h, 6 h, and 12 h can be given. A positive response can be noted as 50% or more recovery of the ptosis in 1 h.[10]
-
Atropine neostigmine (AN) dosage should be stopped if:
After complete recovery from neuroparalysis, the patient should be monitored for recurrence
Side effects in the form of fasciculations or bradycardia noticed
Is no improvement after three doses?
Cobra bite improves with AN and few Nilgiri Russell’s viper bites victims may also improve with this regimen
One dose of ‘AN’ injection should be given before transferring to the higher centre.
Krait bite does not respond to ASV. As it affects presynaptic fibres, gives inj. calcium gluconate 10 ml IV slowly over 5–10 min every 6 hourly and continues till neuroparalysis recovers which may last for 5–7 days, as calcium acts as a neurotransmitter presynaptically.
ASV reactions
Adverse drug reactions range from mild (rash, diaphoresis, etc.) to severe, like anaphylaxis.[25] Reactions can be seen in 5.6–56% of recipients, from which 10–15% being moderate to severe.[29,30] It can present as follows:
Early anaphylaxis reaction
It is usually seen within a few minutes to 180 min after starting antivenom, the patient begins to itch (often over the scalp) and develops urticaria, dry cough, fever, nausea, vomiting, abdominal colic, diarrhoea, and tachycardia. A minority of these patients may develop hypotension, bronchospasm, and angio-oedema, which can be life threatening. As soon as these signs are noted, antivenom administration must be temporarily suspended and epinephrine (adrenaline [0.1% solution, 1 in 1000, 1 mg/ml]) given by intramuscular injection. Antihistamines (e.g., diphenhydramine 25–50 mg intravenous) and intravenous steroids are also indicated.
Pyrogenic (endotoxin) reactions
It usually develops after 1–2 h of treatment, the patient develops shaking chills (rigors), fever, vasodilatation, and a fall in blood pressure. Pyrogen contamination during the manufacturing process may be responsible for this. Physical cooling (remove clothing and tepid sponging with fanning) and an antipyretic is given. Intravenous fluids are indicated for hypovolaemia.
Late (serum sickness type) reactions
The patient may develop fever, nausea, vomiting, diarrhoea, itching, recurrent urticaria, arthralgia, myalgia, lymphadenopathy, periarticular swellings, mononeuritis multiplex, proteinuria with immune complex nephritis, and rarely encephalopathy, usually 1–12 (mean 7) days after treatment. A 5-day course of oral antihistamine can be tried and patients who fail to respond within 24–48 h should be given a 5-day course of prednisolone.
After recovery from the anaphylactic or pyrogenic reaction, the indications for antivenom therapy should be critically reexamined. Intravenous administration should be cautiously resumed until the total dose has been given, if antivenom is still indicated.
Victims who arrive late
Late arrival to a hospital, several days after the bite usually with acute renal failure, is a common problem faced in our country. ASV should be given if coagulopathy still persists, otherwise, treat renal failure only. One dose of 8–10 vials of ASV should be given in neurotoxicity to ensure that no unbound venom is present. However, respiratory support is seldom required at this stage.
Local wound management
Prophylactic antibiotics are indicated if signs of infection are present, cultures and sensitivities of the wound and blood should be obtained to target the offending bacteria.[16] Local tissue inflammation signs such as pain, induration, and numbness can potentially mimic compartment syndrome.[19,24] The role of measurements of elevated intracompartmental pressure for confirmation of compartment syndrome and the need for a fasciotomy is unclear.[25,31,32]
PREVENTION OF SNAKEBITE
Raising community awareness about the prevention of snakebites is the most effective strategy for reducing snakebite morbidity and mortality. Some of the recommendations given by the WHO are to educate children and young adults about the occupational hazards of snakebite, encourage the use of footwear, long pants/trousers, improved lighting, using torches when walking outside, proper lighting in and around houses, encourage people to sleep on a bed and under a well tucked-in mosquito net and provision of buffer zone between fields and housing areas.[28] Furthermore, for the prevention of mortality and reducing morbidity, local quacks should be educated about the hazards and treatment so that they can refer patients to a proper health facility at the earliest.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Guidelines for the Management of Snake-Bites. 2010. Geneva: World Health Organization; Available from: https://www.who.int/snakebites/resources/9789290225300/en [Last accessed on 2021 Dec 02]
- [Google Scholar]
- Premonitory signs and symptoms of envenoming by common krait (Bungarus caeruleus) Trop Doct. 2014;44:82-5.
- [CrossRef] [PubMed] [Google Scholar]
- Snake Bite Envenomation. 2019. Geneva: World Health Organization; Available from: https://www.who.int/news-room/fact-sheets/detail/snakebiteenvenoming [Last accessed on 2021 Dec 02]
- [Google Scholar]
- Snakebite mortality in India: A nationally representative mortality survey. PLoS Negl Trop Dis. 2011;5:e1018.
- [CrossRef] [PubMed] [Google Scholar]
- Trends in snakebite deaths in India from 2000 to 2019 in a nationally representative mortality study. eLife. 2020;9:e54076.
- [CrossRef] [PubMed] [Google Scholar]
- Snakes in Maharashtra. 2018. Available from: https://wwwtreksandtrails.com [Last accessed on 2021 Dec 01]
- [Google Scholar]
- Beyond the 'big four': Venom profiling of the medically important yet neglected Indian snakes reveals disturbing antivenom deficiencies. PLoS Negl Trop Dis. 2019;13:e0007899.
- [CrossRef] [PubMed] [Google Scholar]
- Harrison's Principles of Internal Medicine (20th ed). New York: McGraw-Hill Education; 2018.
- [Google Scholar]
- Standard Treatment Guidelines. Management of Snake Bite, Quick Reference Guide. India: Ministry of Health and Family Welfare, Government of India; 2016.
- [Google Scholar]
- The treatment of snake bites in a first aid setting: A systematic review. PLoS Negl Trop Dis. 2016;10:e0005079.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment of snakebite in Australia: The current evidence base and questions requiring collaborative multicentre prospective studies. Toxicon. 2006;48:941-56.
- [CrossRef] [PubMed] [Google Scholar]
- Tourniquet inefective-ness to reduce the severity of envenoming after Crotalus durissus snake bite in Belo Horizonte, Minas Gerais, Brazil. Toxicon. 1998;36:805-8.
- [CrossRef] [Google Scholar]
- Effects of a negative pressure venom extraction device (Extractor) on local tissue injury after artificial rattlesnake envenomation in a porcine model. Wilderness Environ Med. 2000;11:180-8.
- [CrossRef] [Google Scholar]
- The effect of an electrical current on snake venom toxicity. J Wilderness Med. 1992;3:48-53.
- [CrossRef] [Google Scholar]
- The current concepts in management of animal (dog, cat, snake, scorpion) and human bite wounds. J Trauma Acute Care Surg. 2015;78:641-8.
- [CrossRef] [PubMed] [Google Scholar]
- Suction for venomous snakebite: A study of “mock venom” extraction in a human model. Ann Emerg Med. 2004;43:181-6.
- [CrossRef] [Google Scholar]
- Management of snakebites in the upper extremity. J Hand Surg Am. 2019;44:137-42.
- [CrossRef] [PubMed] [Google Scholar]
- The epidemiology, clinical course, and management of snakebites in the North American snakebite registry. J Med Toxicol. 2017;13:309-20.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnosis and management of compartmental syndromes. J Bone Joint Surg Am. 1980;62:286-91.
- [CrossRef] [Google Scholar]
- Venomous snakebites in the United States: Management review and update. Am Fam Physician. 2002;65:1367-74.
- [Google Scholar]
- Surgical considerations for pediatric snake bites in low-and middle-income countries. World J Surg. 2019;43:1636-43.
- [CrossRef] [PubMed] [Google Scholar]
- Snake bite: When the human touch becomes a bad touch. Toxins (Basel). 2018;10:170.
- [CrossRef] [PubMed] [Google Scholar]
- Acute adverse events associated with the administration of crotalidae polyvalent immune fab antivenom within the North American snakebite registry. Clin Toxicol (Phila). 2018;56:1115-20.
- [CrossRef] [PubMed] [Google Scholar]
- Points and pearls: Emergency department management of North American snake envenomations. Emerg Med Pract. 2018;20(Suppl 19):1-2.
- [Google Scholar]
- Snake Bites New Jersey. 2020. United States: Merck & Co Inc.; Available from: https://www.merckmanuals.com/home/injuries-and-poisoning/bites-and-stings/snakebites [Last accessed on 2021 Dec 03]
- [Google Scholar]
- Rational use of anti-snake venom (ASV): Trial of various regimens in hemotoxic snake envenomation. J Assoc Physicians India. 2004;52:788-93.
- [Google Scholar]
- Adverse drug reaction profile of anti-snake venom in a rural tertiary care teaching hospital. J Young Pharm. 2013;5:41-5.
- [CrossRef] [PubMed] [Google Scholar]
- Compartment syndrome of the lower extremity In: Mauffrey C, Hak DJ, Martin IM, eds. Compartment Syndrome: A Guide to Diagnosis and Management. Cham: Springer; 2019. p. :67-81.
- [CrossRef] [PubMed] [Google Scholar]
- Compartment syndrome in the hand following an adder bite. J Hand Surg Br. 2005;30:434-5.
- [CrossRef] [PubMed] [Google Scholar]






