Translate this page into:
Acute Myocardial Infarction in a Patient of Polycythaemia Vera
*Corresponding author: Vinod Khandait, Department of Medicine, Government Medical College, Nagpur, Maharashtra, India. khandaitvinod@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Khandait V, Bhujle SS, Bagchi N. Acute myocardial infarction in a patient of polycythaemia Vera. Vidarbha J Intern Med 2022;32:59-62.
Abstract
Polycythaemia Vera is the most common form of myeloproliferative neoplasm.The median age of diagnosis is 60 years. Atherosclerosis is the most common cause of myocardial infarction, however other causes too should be looked for in the patients. Here, we present a case of 62 year old female who was a known case of hypertension and Ischemic heart disease on regular medications. She came with complaints of chest pain for the past 2 days. ECG showed NSTEMI with raised cardiac enzymes. She developed left side hemiparesis after admission, and the CT head showed acute infarct in right frontal lobe. She was started on treatment for NSTEMI and cerebrovascular event. Her CBC showed Hb-17.1g/dl, TLC-51600/mm3 and platelet count-625000/mm3, hence we suspected her to have polycythemia Vera with trilineage involvement. Serum erythropoietin was normal. JAK2(V617) profile was positive for homozygous (TT) mutation. Bone marrow biopsy findings were suggestive of trilineage hyperplasia. She was diagnosed as a case of polycythemia Vera. Our patient came into the age group wherein various risk factors for ischemic heart disease could be present like hyperlipidaemia, hypertension, diabetes mellitus but a careful look out for other causes too should be kept in mind.
Keywords
Acute Myocardial Infarction
Polycythemia Vera
INTRODUCTION
Polycythaemia Vera is the clonal haematopoietic stem cell disorder in which phenotypically normal red cells, granulocytes and platelets accumulate in the absence of a recognisable physiological stimulus. It is the most common form of myeloproliferative neoplasm. The median age of diagnosis is 60 years, though the disorder can occur in all age groups.[1]
CASE REPORT
A 62-year-old female, resident of Chandrapur district, Maharashtra, was brought by relatives to medicine OPD, with complaints of chest pain for 2 days, retrosternal in location, crushing type, and non-radiating. She was a known case of systemic hypertension with ischaemic heart disease on regular medications for 2 years.
On general examination, she was afebrile, conscious oriented, pulse rate 80/min, regular, good volume, BP-140/90 mmHg, and saturation 98% on room air. On abdominal palpation, there was moderate splenomegaly. Rest systemic examination was WNL. On admission, she developed sudden onset left-sided haemiparesis. Her electrocardiogram on presentation showed LVH with T wave inversions in pre-cordial leads V1– V6. Her troponin I was positive. Her investigations revealed elevated total WBC count, haemoglobin and platelet count.
| 4 September 2021 | 7 September 2021 | |
|---|---|---|
| Hb | 16.2 | 17.1 |
| Haematocrit | 54 | 56 |
| TLC | 46,400 | 51,600 |
| PLT | 682,000 | 625,000 |
| Protein | 6.8 | 6.4 |
| Bilirubin | 0.5 | 0.4 |
| ALP | 188 | 123 |
| AST | 35 | 32 |
| ALT | 36 | 35 |
| UREA | 22 | 19 |
| CREAT | 1.0 | 0.9 |
| TOT CHOL | 91.4 | |
| HDL | 47.5 | |
| LDL | 19.22 | |
| TRIG | 123.4 |
Her serum for HIV, HBSAg and anti-HCV was negative. Serum erythropoietin was 8.82 mIU/ml (normal). JAK2 (V617) profile was positive for homozygous (TT) mutation. USG (abdomen and pelvis) revealed gross splenomegaly with a benign cyst of the right ovary. CT head plain revealed ill-defined hypodensity involving the right middle frontal gyrus without any mass effect. Bone marrow biopsy findings suggested trilineage hyperplasia, occasional fine fibres with focal fine network Grade 1 (on reticulin stain), 2D ECHO-ischaemic heart disease, RWMA present, anterior, anterolateral, and anteroseptal hypokinesia and LVEF 40%. T wave inversion in in prcordial leads V1-V6 [Figure 1]. Coronary angiogram showed single-vessel disease of LAD (type 3 ostioproximal 70% stenosis).
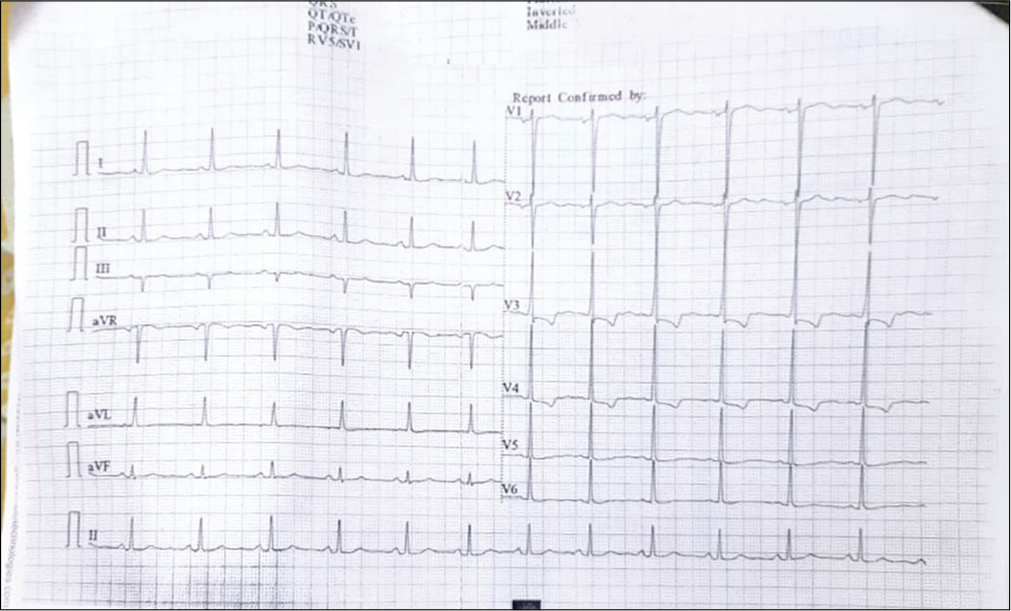
- Electrocardiogram on presentation.
Bone Marrow examination was suggestive of Panmyelosis [Figure 2]. The patient was given 5 days of low-molecular-weight heparin s.c injections, dual antiplatelet agents, and statins for persistent chest pain and CVE. Antihypertensive medications were continued. She showed gradual improvement. She followed up with the haemato-oncologist and was prescribed tablet hydroxyurea 500 mg TDS and tablet aspirin 75 mg OD for 1 month. She was later discharged on request.

- Bone marrow biopsy report.
DISCUSSION
The incidence of polycythaemia Vera is 2.3 per 100,000 persons per year.[2] Isolated thrombocytosis, leucocytosis or splenomegaly may be the initial presenting manifestation of polycythaemia Vera, but most often the disorder is first recognised by the incidental discovery of high haemoglobin, haematocrit or red cell count.
Coronary events are not uncommon during the course of polycythaemia Vera. Rosie et cetera all followed 149 patients diagnosed with polycythaemia Vera for 10 years and found that 11.4% had a myocardial infarction.[3] Another report concluded that 4% of all patients with the myeloproliferative disorder die from myocardial infarction.[4]
The incidence of thrombotic complications in PV patients ranges from 13 to 60%. The presence of traditional cardiac risk factors further increases the risk of thrombosis, especially coronary artery thrombosis.
The 2016 WHO criteria for diagnosis of polycythaemia Vera include the following:
Major criteria
Increased haemoglobin level (>16.5 g/dL in men or >16 g/dL and woman), haematocrit (>49% in men or >48% in women) or other evidence of increased red cell volume
Bone marrow biopsy showing hypercellularity for age with trilineage growth (panmyelosis) includingerythrocytes, granulocytic and megakaryocytic proliferation with pleomorphic and mature megakaryocytes
JAK2V617 or JAK2 exon 12 mutation.
Minor criteria
Serum erythropoietin level below the reference range for normal.
The diagnosis of polycythaemia Vera requires the presence of all three major criteria or the presence of the first two major criteria with the minor criteria.[5] Our patient here satisfied the major as well as minor criteria.
Uncontrolled erythrocytes cause hyperviscosity, leading to neurologic symptoms such as vertigo, tinnitus headache, visual disturbances, and transient ischaemic attacks. Systolic hypertension is also a feature of red cell mass elevation. In some patients, venous or arterial thrombosis may be the presenting manifestation of polycythaemia Vera. Any vessel can be affected; but cerebral, cardiac, and mesenteric muscles are commonly involved.
Excessive activation of the JAK/STAT pathway and platelet turnover is associated with thrombosis. Due to accelerated platelet circulation, immature platelets are released into the blood.[6] These compounds have a powerful haemostatic effect. Furthermore, enhanced platelet production of 5-hydroxytryptamine, β-thromboglobulin and platelet factor 4 due to JAK2 gene mutation also increases the risk of thrombosis.[7] Compared with JAK2V617F mutation-negative patients, positive patients have higher white blood cell and platelet counts and an increased incidence of thrombosis.[8] In patients with PV, arterial thrombi form due to platelet activation, adhesion and aggregation. Proposed mechanisms of thrombogenesis are multifactorial but involve increased shear stress due to high haematocrit leading to vessel wall inflammation and closer contact of platelets to the endothelium, leading to platelet activation.
Such patients are prone to intraprocedural thrombosis. The use of intracoronary eptifibatide or abciximab inhibits platelet aggregation by reversibly blocking the GP IIb/IIIa receptor, decreasing fibrinogen and Von Willebrand factor-mediated platelet activation.[9] Intracoronary eptifibatide doses can be up to 1000 times that delivered intravenously and can disaggregate the thrombus itself.[10] Prior publications have reported intraprocedural phlebotomy to combat thrombus formation. There are no guidelines addressing the acute management of arterial thromboembolism in patients with PV.[11]
Management of polycythaemia Vera is guided by a risk adapted approach that is based on age and history of prior thrombosis. Patients who are <60 years old with no history of thrombosis are classified as a low risk; all others are considered high risk.
Management of low-risk polycythaemia Vera
Phlebotomy to maintain the haematocrit <45%
Low-dose aspirin
Assessment and management of cardiovascular risk factors and counselling to stop smoking.
Management of high-risk polycythaemia Vera
Phlebotomy to maintain the haematocrit <45%
Treatment with low-dose aspirin
Cytoreductive therapy with hydroxyurea or interferon or busulphan
Assessment of cardiovascular risk factors and counselling to stop smoking.
PV patients who undergo PCI are left with having a higher than normal risk of stent thrombosis, although the true risk is unknown, nor do any guidelines exist on the antiplatelet regimen that should be followed. Logically, using more potent P2Y12 inhibitors such as ticagrelor or prasugrel in combination with ASA would lead to a lower stent thrombosis risk than that of clopidogrel. No data exist on the use of anticoagulants in this scenario. Given the propensity for stent thrombosis in these patients, we agree that if the patient can be adequately treated without stent insertion, this would be preferable, but if unavoidable, dual antiplatelet therapy with aspirin and ticagrelor/prasugrel, in addition to hydroxyurea and phlebotomy, would likely result in the least long-term morbidity/mortality and the lowest risk of stent thrombosis.
Median survival of untreated symptomatic patients with polycythaemia Vera[12] has been estimated as 18 months but survival is at least 13 years in patients who were treated.[13] A major cause of death in polycythaemia Vera is disease transformation to post polycythaemia Vera myelofibrosis and/or evolution to acute myeloid leukaemia/ myelodysplastic syndrome.[14]
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Polycythemia vera: A comprehensive review and clinical recommendations. Mayo Clin Proc. 2003;78:174-94.
- [CrossRef] [PubMed] [Google Scholar]
- Acute coronary disease in essential thrombocythemia and polycythemia vera. J Intern Med. 1998;244:49-53.
- [CrossRef] [PubMed] [Google Scholar]
- French group of familial myeloproliferative disorders. Long term follow up of 93 families with myeloproliferative neoplasms: Life expectancy and implications of JAK2V617F in the occurrence of complications. Blood Cells Mol Dis. 2012;49:170-6.
- [CrossRef] [PubMed] [Google Scholar]
- Classification, diagnosis and management of myeloproliferative disorders in the JAK2V617F era. Hematol Am Soc Hematol Educ Program. 2006;2006:240-5.
- [CrossRef] [PubMed] [Google Scholar]
- Recurrent cardiovascular events despite antiplatelet therapy in a patient with polycythemia vera and accelerated platelet turnover. Am J Case Rep. 2017;18:945-48.
- [CrossRef] [PubMed] [Google Scholar]
- Aspirin-responsive, migraine-like transient cerebral and ocular ischemic attacks and erythromelalgia in JAK2-positive essential thrombocythemia and polycythemia vera. Acta Haematol. 2015;133:56-63.
- [CrossRef] [PubMed] [Google Scholar]
- The prevalence of JAK2, MPL, and CALR mutations in Chinese patients with BCR-ABL1-negative myeloproliferative neoplasms. Am J Clin Pathol. 2015;144:165-71.
- [CrossRef] [PubMed] [Google Scholar]
- Intracoronary abciximab and aspiration thrombectomy in patients with large anterior myocardial infarction: The INFUSE-AMI randomized trial. JAMA. 2012;307:1817-26.
- [CrossRef] [PubMed] [Google Scholar]
- Elevating local concentrations of GPIIb-IIIa antagonists counteracts platelet thrombus stability. J Thromb Thrombolysis. 2013;36:31-41.
- [CrossRef] [PubMed] [Google Scholar]
- Polycythaemia vera presenting as ST-elevation myocardial infarction. Heart Lung Circ. 2005;14:51-3.
- [CrossRef] [PubMed] [Google Scholar]
- Complications and causes of death in polycythaemia vera. Acta Med Scand. 1962;172:513-23.
- [CrossRef] [PubMed] [Google Scholar]
- Long-term survival and blast transformation in molecularly annotated essential thrombocythemia, polycythemia vera, and myelofibrosis. Blood. 2014;124:2507-13. quiz 2615
- [CrossRef] [PubMed] [Google Scholar]
- Higher risk of primary cancers after polycythaemia vera and vice versa. Br J Haematol. 2011;153:283-5.
- [CrossRef] [PubMed] [Google Scholar]






