Translate this page into:
Azathioprine-Induced Anagen Effluvium: A Case Series of Three Cases
*Corresponding author: Shirin Sunil Gawali, Department of Dermatology, Government Medical College Nagpur, Maharashtra, India. 386shirin@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Supekar BB, Chopkar AD, Gawali SS, Mukhi JI, Nama P. Azathioprine-Induced Anagen Effluvium: A Case Series of Three Cases. Vidarbha J Intern Med. 2023;33:95-8. doi: 10.25259/VJIM_8_2023
Abstract
Azathioprine is a commonly used drug in dermatology for immunobullous diseases, vitiligo, and lichen planus alopecia areata. Azathioprine is rarely known to cause the acute onset of anagen effluvium (AE) that may precede myelosuppression associated with it. We report a series of three cases of azathioprine-induced AE. All three of our cases were females who were treated with azathioprine for non-dermatological conditions such as neuromyelitis optica, idiopathic thrombocytopenia, and optic neuritis. We report this series mainly due to its rarity.
Keywords
Anagen effluvium
Azathioprine
Myelosuppression
INTRODUCTION
Anagen effluvium (AE) is the abrupt loss of hairs that are in their growing phase (anagen) due to events that impair the mitotic or metabolic activity of hair follicles.[1] The term AE can be misleading due to abnormal hairs being broken off rather than being shed. Azathioprine has also rarely been implicated with AE. It is reversible, as hair growth appears after discontinuing the offending agent.
CASE REPORT
Case 1
A 42-year-old female was admitted to the medicine ward and referred to our department with complaints of sudden onset of hair loss for 21 days without any associated symptoms. She had a known case of neuromyelitis optica for two years and was started on Tab Azathioprine in a dose of 50 mg once daily for the past 1.5 months. There was no history of any other drug intake during this period of treatment. No similar history in the past. On examination, diffuse non-cicatricial alopecia was seen all over the scalp but predominantly over the vertex [Figures 1a-d]. Hair was effectively dislodged in clumps while performing a hair pull test. Microscopic examination of hair revealed anagen hair bulb [Figure 2a]. Trichoscopy of vertex region was performed using Escope dermoscope, ×10 magnification with polarised mode. Trichoscopy revealed numerous black dots [Figure 2b], multiple hair shaft residues [Figure 2c], exclamation hair [Figure 2c] suggestive of Anagen effluvium [Figures 2b-d]. A complete hemogram revealed severe myelosuppression. (Haemoglobin [Hb] – 7.5 g/dL, platelets – 3000/cumm, and white blood cell [WBC] – 1200/cumm) and azathioprine was withheld. This case was reported to India’s pharmacovigilance program (PVPI) (report no. 300542341). Based on the World Health Organization’s health coverage (WHO-UHC) scale, this adverse drug reaction (ADR) was probable. The patient was then treated with injection filgrastim, antibiotics, and doses of steroid were given; following this, there was spontaneous improvement in blood counts, leukocyte counts improved to 4700/cumm, platelets 78000/cumm and Hb 10 g/dL. Moreover, mild regrowth of hairs was seen at two months after the discontinuation of therapy.

- (a) Extensive non-cicatricial alopecia involving frontal, (b) occipital , (c and d) parietal region of both right and left side.
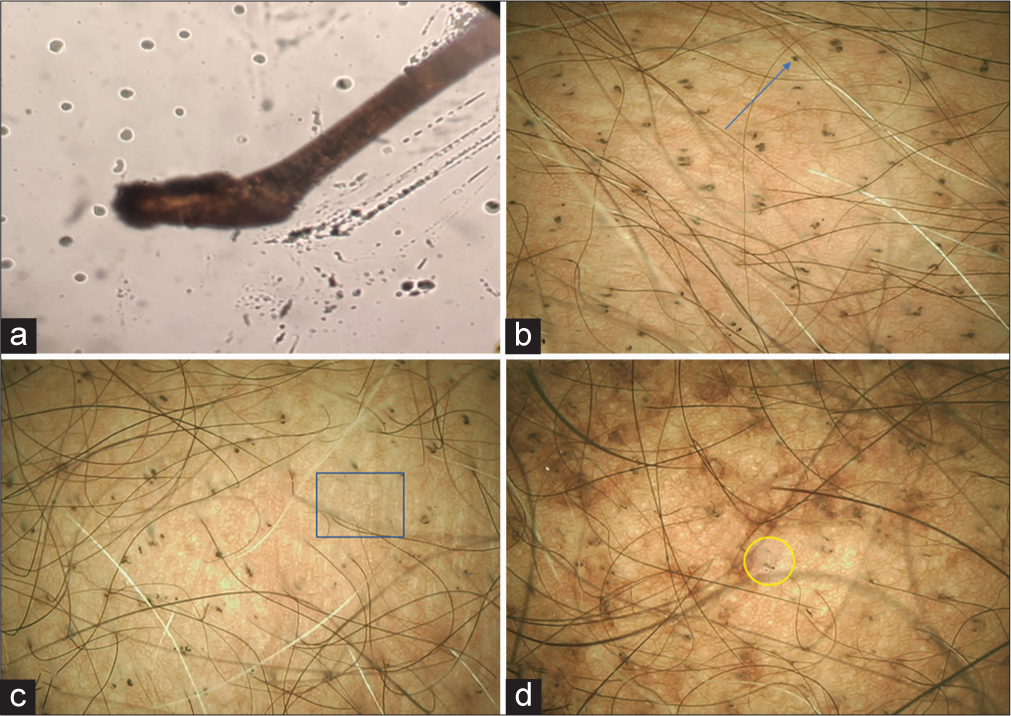
- (a) Anagen hair bulb as visible in hair microscopy (40× magnification). (b) black dot (blue arrow), (c) multiple hair shaft residue (blue box), (d) exclamation mark hair (yellow circle), suggestive AE on trichoscopy. (e-scope 10× magnification).
Case 2
A 30-year-old female patient presented to us with complaints of sudden onset of diffuse hair loss from the scalp with the formation of clumps of hairs on combing without any symptoms for 15 days. The patient was a known case of idiopathic thrombocytopenic purpura and was being treated with Tab. Azathioprine in a dose of 50 mg OD for 30 days. There was no history of any other drug being used for treatment. On examination, diffuse non-scarring alopecia was more prominently seen over the occipital and vertex regions, and the hair pull test was positive [Figures 3a-d].
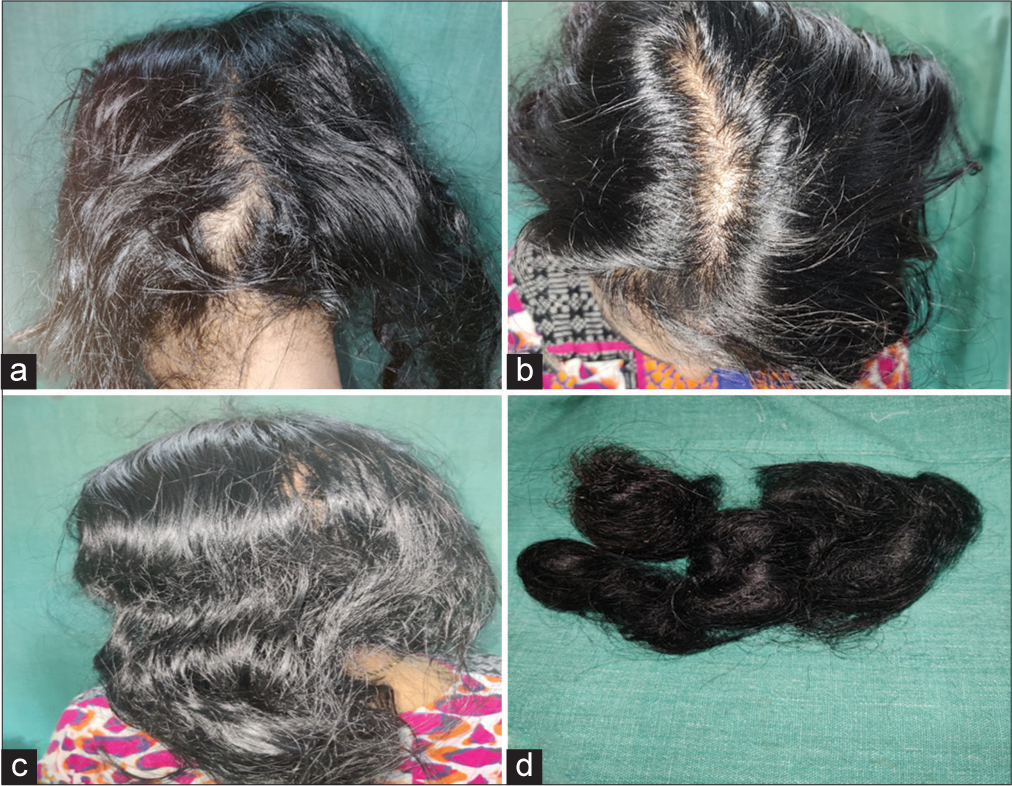
- (a) Diffuse thinning of hair in a treated case of idiopathic thrombocytopenic purpura on occiput, (b) vertex, (c) parietal aspect, (d) bunch of hairs collected by patient.
The hair loss was accompanied by myelosuppression (Hb – 7 gm/dL, WBC – 2900/cumm, and platelets – 2280/cumm). Based on the findings mentioned above, a diagnosis of azathioprine-induced AE with myelosuppression was reached; therefore, azathioprine was discontinued. This case was reported to PVPI, and based on the WHO–UHC scale, this ADR reaction was probable (report no. 300542403). After two months, the patient had regrowth of normal anagen hair, and improvement in blood counts was seen.
Case 3
A 35-year-old female was admitted to the medicine ward for complaints of recurrent episodes of fever and oral ulcers. The patient was then referred to us for complaints of diffuse hair loss for 20 days. On inquiry, there was a history of consumption of tablet azathioprine in the dose of 50 mg twice daily for two months for treatment of optic neuritis from a private hospital. Cutaneous examination revealed diffuse non-cicatricial alopecia involving the occipital, frontal, bilateral parietal, and temporal region, with a positive hair pull test [Figures 4a-d]. Rest cutaneous examination was normal. Trichoscopy, as well as routine microscopic examination of hairs, showed similar findings as mentioned in the above cases. Complete hemogram was normal except for hypoplastic anaemia (Hb – 7.8 g/dL, WBC – 1900 cumm, platelet – 88,000 cumm, and bone marrow aspiration suggestive of hypoplastic anaemia). Based on the above findings, a diagnosis of azathioprine-induced AE was considered. Azathioprine was then withheld, and the case was reported to the PVPI. Based on the WHO-UHC scale, the reaction was considered as a probable ADR. The patient was started on symptomatic treatment but was then lost to follow-up due to the COVID-19 pandemic. Thiopurine methyltransferase was not done in all cases due to financial constraints.
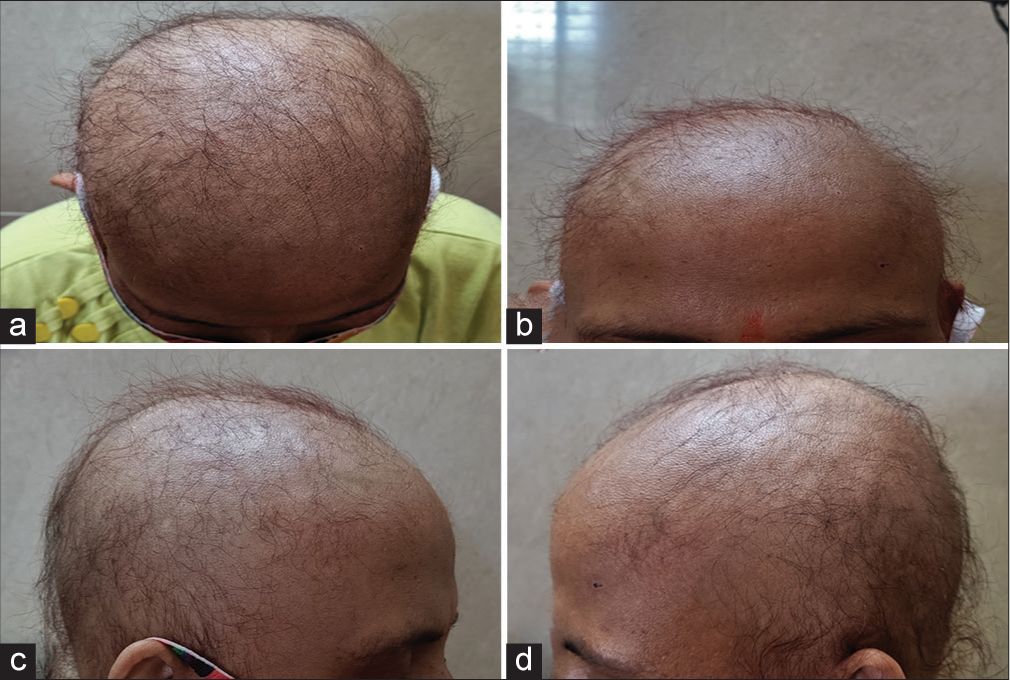
- Alopecia in a case of optic neuritis treated on azathioprine as seen on (a) vertex, (b) frontal, (c) and (d) parietal aspect of each side.
DISCUSSION
AE is a form of reversible, non-scarring alopecia mainly affecting the hairs in the growing phase due to chemotherapeutic drugs. Approximately 90% of hairs taken from a normal scalp are anagen hairs. This is a period of epithelial proliferation, in which bulb matrix cells undergo mitosis and proliferation to form the hair shaft. Severe insult to the hair bulb or hair matrix in the form of medications, toxin exposure, or radiation that causes a cessation of this mitotic activity can cause damage to the hair shaft resulting in weakening of the partially keratinised, proximal portion of the hair shaft, resulting in narrowing and subsequent breakage within the hair canal and even complete failure of hair formation.[2] Fortunately, quiescent bulge stem cells are spared, thus re-initiating follicle growth within 1–3 months and making it a reversible process.[3] Many chemotherapeutic drugs, such as antimetabolites, alkylating agents, and mitotic inhibitors, have been implicated in the pathogenesis of AE. Doxorubicin, nitrosoureas, and cyclophosphamide have been implicated too. Several other drugs, such as isoniazid, levodopa, colchicine, cyclosporine, and toxic heavy metals, are also included.[4] Azathioprine is only formally approved by the United States Food and Drug Administration for its use in organ transplantation and severe rheumatoid arthritis. Dermatologists most likely prescribe it as a steroid-sparing agent used in the management of immunobullous diseases, neutrophilic, connective tissue, photodermatosis, and papulosquamous disorders.[5] Azathioprine is less commonly associated with maculopapular, urticarial, or purpuric ADRs such as sweet syndrome, Erythema nodosum, small vessel vasculitis, and acute generalised exanthematous pustulosis.[6] Dose-dependent side effects such as myelosuppression, infection, nausea, and vomiting hepatotoxicity are well-characterised manifestations and can occur at any time during the therapy.[7]
Dermatological problems are more frequent in organ transplantation patients than in non-transplantation patients on azathioprine.[8] Common skin problems include alopecia, venereal, zoster, increased skin colour, and malignancy. Azathioprine-induced AE usually occurs after 2–4 weeks of drug intake, although uncommon, has been reported previously [Table 1]. However, the degree of azathioprine-induced alopecia usually starts within one month of the therapy, as seen in our cases as well. The degree of hair loss varies from person to person. It depends on a number of factors, such as specific agents, the dose and duration of therapy, and the route of administration. Being non-scarring alopecia, the follicular ostia are intact with no evidence of erythema, scaling, pigmentation, or scarring. Hair loss can be diffuse or patchy at the onset, depending on the amount of hair in the active anagen phase.
| S. No. | Case report | Age/sex | Dose | Description | Reversal |
|---|---|---|---|---|---|
| 1. | Joshi and Singh (2010)[12] | 54/F | 50 mg once daily | Interstitial lung disease | -- |
| 2. | Balasubramanian et al. (2015)[13] | 30/M | 50 mg once daily | Cutaneous leukocytoclastic vasculitis | 2 months |
| 3. | Sonthalia and Daulatabad (2016)[2] | 30/F | 50 mg once daily | Vitiligo Vulgaris | 6 months |
| 4. | Bhokare (2017)[14] | 20/F | 50 mg once daily | Vitiligo | 2 months |
| 5. | Wankhade et al. (2018)[4] | 20/F | 50 mg once daily | Pemphigus Vulgaris | 2 months |
| 6. | Sharma et al. (2017)[15] | 35/F | 50 mg BD | Atopic dermatitis | 4 months |
| 7. | Our cases | 42/F | 50 mg once daily | Neuromyelitis Optica | 2 months |
| 30/F | 50 mg once daily | Idiopathic thrombocytopenic purpura | 2 months | ||
| 35/F | 50 mg twice daily | Optic neuritis | Lost to follow up |
Trichoscopic evaluation of AE includes black dots, yellow dots, Pohl-Pinkus constriction and tapering hairs, and peripilar signs.[9] Features like Tupiloid hair have also been described as a specific finding of AE.[10] Other findings include exclamation hair, multiple hair shaft residue, and black dots, as described in this case. Various case reports have demonstrated this side effects of azathioprine induced anagen effluvium as a marker of myelosuppression.[12-15]
We report this case mainly because azathioprine is a commonly used drug in dermatology; one should be aware of its side effects as hair loss induced by this drug also can serve as an impending marker for azathioprine-induced myelosuppression as seen in our cases.
AE is usually reversible; the goal is to prevent or shorten the period of alopecia. The use of wig hair pieces can be done. Multiple cooling systems such as ice turban, gel packs, cool caps, thermocirculators, and room air conditioners can be used to induce scalp hypothermia. AS101 and minoxidil were able to reduce the severity or shorten the duration of chemotherapy-induced alopecia.[11]
CONCLUSION
Azathioprine remains as an important immunosuppressive agent in the world of medicine however side effects like anagen effluvium proves to be an important early marker of myelosuppression which should be known for monitoring the therapeutic response of the drug.
Ethical approval
The research/study complied with the Helsinki Declaration of 1964.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Hair Growth Disorders In: Wolff K, Goldsmith LA, Katz SI, Gilchrest BA, Zller AS, Leffell DJ, eds. Fitzpatrick’s Dermatology in General Medicine Vol 2. (7th ed). New York: McGraw-Hill; 2008. p. :753-77.
- [Google Scholar]
- Azathioprine-Associated Anagen Effluvium. Indian J Dermatol Venereol Leprol. 2016;82:322-4.
- [CrossRef] [PubMed] [Google Scholar]
- Anagen Effluvium. Indian J Dermatol Venereol Leprol. 2013;79:604-12.
- [CrossRef] [PubMed] [Google Scholar]
- Azathioprine Induced Anagen Effluvium and Its Association with Myelosuppression. Iran J Dermatol. 2018;21:68-70.
- [Google Scholar]
- Azathioprine In: Wolverton SE. editor. Comprehensive Dermatologic Drug Therapy. Philadelphia, PA: Saunders; 2001. p. :165-79.
- [Google Scholar]
- The Cutaneous and Systemic Manifestations of Azathioprine Hypersensitivity Syndrome. J Am Acad Dermatol. 2011;65:184-91.
- [CrossRef] [PubMed] [Google Scholar]
- Azathioprine Hypersensitivity: Report of Two Cases and Review of the Literature. J Nephrol. 2003;16:272-6.
- [Google Scholar]
- The Role of Genetic Variation in Thiopurine Methyltransferase Activity and the Efficacy and/or Side Effects of Azathioprine Therapy in Dermatologic Patients. Arch Dermatol. 1995;131:193-7.
- [CrossRef] [Google Scholar]
- Trichoscopy in Anagen Effluvium: Extensive Peripilar Sign. Our Dermatol Online. 2017;8:493-4.
- [CrossRef] [Google Scholar]
- Tulipoid Hair: Anagen Effluvium Marker! Int J Trichology. 2018;10:188-90.
- [CrossRef] [PubMed] [Google Scholar]
- The Protective Role of the Immunomodulator AS101 against Chemotherapy-induced Alopecia Studies on Human and Animal Models. Int J Cancer. 1996;65:97-103.
- [CrossRef] [Google Scholar]
- Plica Neuropathica (Plica Polonica) Following Azathioprine-Induced Pancytopenia. Int J Trichology. 2010;2:110-2.
- [CrossRef] [PubMed] [Google Scholar]
- An Interesting Case Report of Azathioprine-Induced Anagen Effluvium. Indian J Dermatol. 2015;60:324.
- [CrossRef] [PubMed] [Google Scholar]
- Azathioprine-Induced Alopecia as an Early Clinical Marker of Its Myelotoxicity. Indian J Drugs Dermatol. 2017;3:40-1.
- [CrossRef] [Google Scholar]
- Azathioprine Induced Anagen Effluvium: A Rare Case Report. Int J Res Dermatol. 2017;3:300-2.
- [CrossRef] [Google Scholar]






