Translate this page into:
Jejunal Gastrointestinal Stromal Tumour Presenting with Profound Lower Gastrointestinal Bleeding
*Corresponding author: Pankaj Janardhan Nawghare, Department of Gastroenterology, Asha Hospital, Nagpur, Maharashtra, India. pankaj9ghare21@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Nawghare PJ, Agrawal S, Somkuwar A, Agrawal R. Jejunal Gastrointestinal Stromal Tumour Presenting with Profound Lower Gastrointestinal Bleeding. Vidarbha J Intern Med. 2023;33:104-6. doi: 10.25259/VJIM_7_2024
Abstract
Gastrointestinal stromal tumours (GISTs) are uncommon malignant mesenchymal neoplasms that affect the gastrointestinal (GI) system. We describe the case of a 45-year-old man who arrived at the emergency room with haemodynamic instability and profound lower GI haemorrhage. Despite early resuscitation efforts, bleeding continued, necessitating a further investigation. A sizable solid-cystic lesion in the jejunum that was heterogeneously enhancing was seen on computed tomography abdominal angiography. Urgent surgical exploration was performed, uncovering a large mid-jejunal tumour that was successfully removed, restoring haemodynamic stability. A histopathological analysis verified the presence of GIST, an uncommon jejunal occurrence. This case study emphasises the necessity of investigating GIST as a differential diagnosis in individuals presenting with significant lower GI bleeding, even if it is uncommon for their age. Surgical excision remains the cornerstone of care for localised, non-metastatic GISTs, especially in situations of severe bleeding when radiologic therapies are not possible due to haemodynamic instability.
Keywords
Jejunal
Young patient
Bleeding
Urgent surgery
Gastrointestinal stromal tumour
INTRODUCTION
Gastrointestinal stromal tumours (GISTs) are rare tumours of the gastrointestinal (GI) tract. GISTs, initially reported by Mazur and Clark in 1983, arise from the interstitial cells of Cajal.[1] These tumours can develop anywhere in the GI tract; however, jejunal GIST is extremely rare.[2,3] The majority of these tumours are asymptomatic. GIST most frequently presents as intermittent GI bleeding (42%); however, major bleeding that is life-threatening and requires immediate medical attention is an uncommon presentation.[4] We have reported a massive lower GI bleed in a young patient, detected to have a jejunal GIST and managed with urgent surgical intervention.
CASE REPORT
A 32-year-old male presented to the emergency department with a history of bleeding per rectum for five days. Bleeding per rectum was profuse, continuous, fresh red blood and associated with fainting and dizziness. There was no history of hematemesis, abdominal pain, or abdominal lumps. The patient denied any addictions. His previous medical history was unremarkable. On admission, the patient was hypotensive with a blood pressure of 86/50 mmHg, a pulse of 126 beats/min, a temperature of 37°C and an oxygen saturation of 100% on room air. On palpation, the abdomen was soft with no lumps or organomegaly, and rectal examination revealed fresh red blood. On admission, his lab results showed haemoglobin of 3.4 g/dL, a white cell count of 8000/µL, platelets of 74 × 109/L and an mean corpuscular volume (MCV) of 64 fl. His liver function test and international normalised ratio were normal. Despite the initial fluid resuscitation and transfusion of three units of red blood cells, the patient remains unstable with ongoing bleeding per rectum. The patient was taken for computed tomography (CT) abdomen angiography, which showed a 9.8 × 6.2 × 6.1 cm heterogeneously enhancing solid-cystic lesion with non-enhancing intralesional necrotic areas at the level of the umbilicus [Figure 1]. There was no active contrast ooze, no regional lymph node enlargement and no metastatic deposition to other organs.
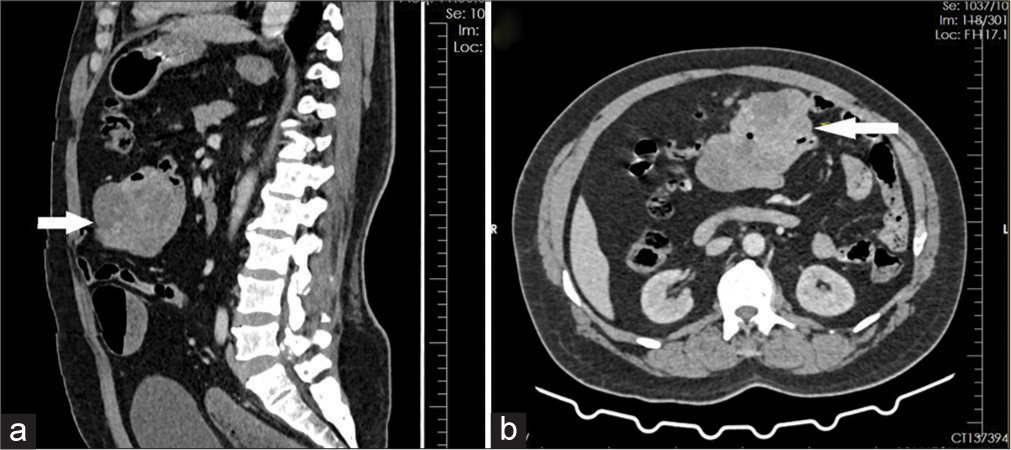
- (a and b) Computed tomography scan angiogram image showing a 9.8 × 6.2 × 6.1 cm heterogeneously enhancing solid-cystic lesion arising from the proximal jejunum (the arrow).
With ongoing resuscitation, he was taken for urgent surgical exploration. The intraoperative findings revealed a large midjejunal mass of about 12 × 7 × 5 cm with an intact serosal covering [Figure 2]. The tumour was resected with a 5 cm clearance margin on each side, and bowel anastomosis was performed side by side.
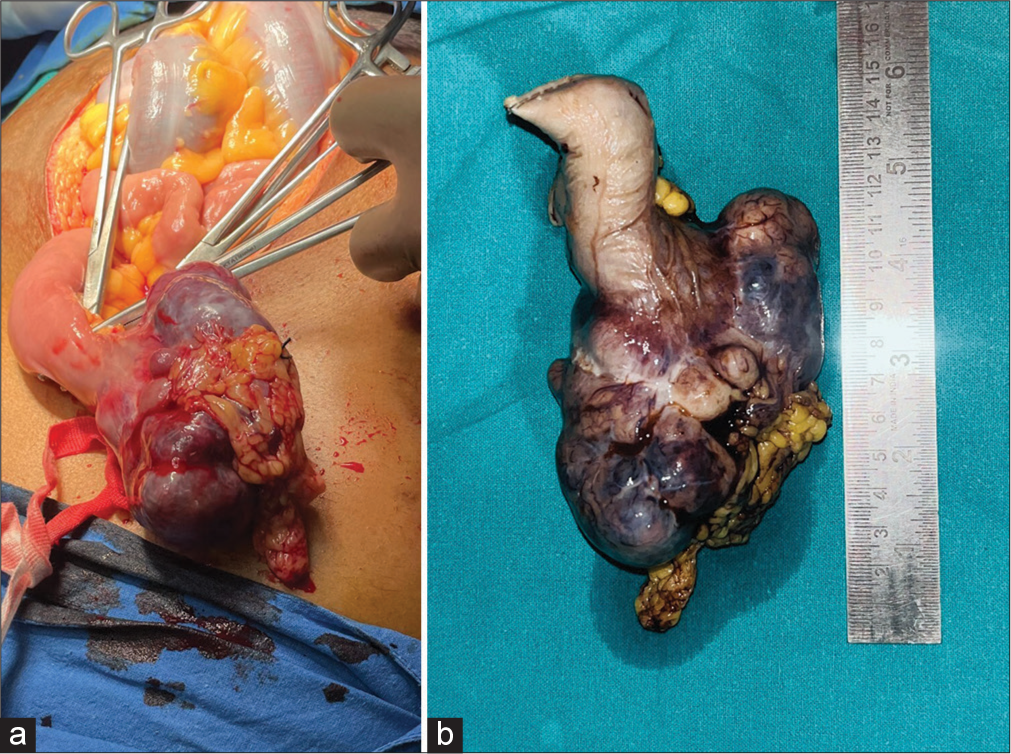
- (a) Intraoperative image and (b) resected specimen showing tumour arising from jejunum measuring 12 × 7 × 5 cm.
The post-operative period was uneventful. Following surgery, haemoglobin levels stabilised and inotropes were gradually stopped after a 24-h period. The patient began oral feeding on the 3rd post-operative day and was discharged on the 6th postoperative day. A gross examination of the resected specimen revealed a submucosal tumour measuring 10 × 6 × 5 cm with areas of haemorrhage. The mucosa surface was ulcerated, but the serosal surface appeared intact without any signs of rupture. Microscopically, the tumour is composed of lobules of intertwining bundles of spindle cells with scanty stroma. On immunohistochemistry, cells were positive for cluster of differentiation 117 (CD117) and discovered on GIST 1 (DOG-1) [Figure 3]. Mitotic count was low (<5/50 high power field [HPF]).
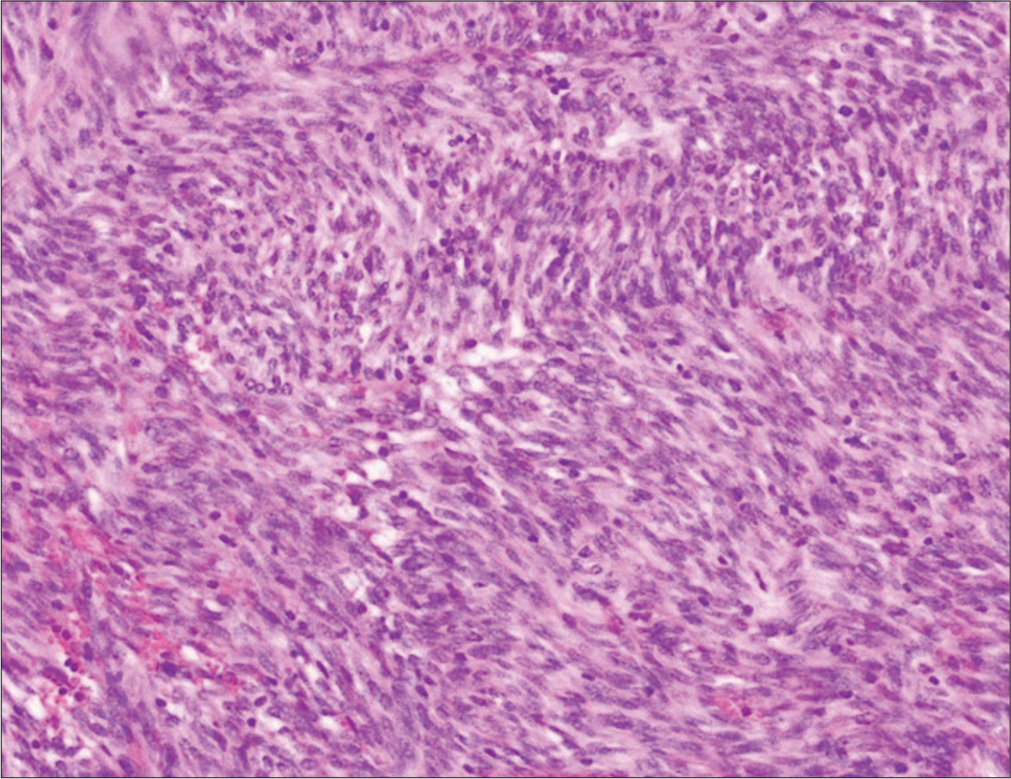
- Histopathology examination showing intertwining bundles of spindle cells.
DISCUSSION
GIST is the most common malignant mesenchymal neoplasm of the GI tract.[5] GIST can occur anywhere along the digestive tract, with higher incidences in the stomach and small intestine.[6-8] Jejunal GISTs are an extremely rare type, accounting for 0.1-3% of all GI tumours.[2,3]
The average age of tumour presentation is 65 years, with a male-to-female ratio of 1:1.[9] GIST is primarily caused by a genetic mutation in the KIT gene, with 95% of cases linked to this gene, while only 3-5% are linked to platelet-derived growth factor receptor alpha.[10] The clinical presentation in GIST is broad and primarily depends on the size of the tumour and its location. Small-sized GISTs, often asymptomatic, pose a challenge for early diagnosis and are frequently discovered incidentally on CT, endoscopy or intraoperatively. The symptoms, which are often non-specific, include a palpable mass, GI bleeding, anaemia, weight loss, vomiting and epigastric pain.[6] Most cases of small intestinal GIST present with chronic occult intraluminal GI bleeding.[4] Although some reports of massive, life-threatening bleeding from a small intestinal bleed have been reported. Our patient had a massive lower GI bleed and was haemodynamically unstable at presentation. Although endoscopic examination is usually the first modality to determine the source of bleeding, we proceed with CT angiography as the patient could not be stabilised despite resuscitation. The patient was successfully managed with surgical excision of the tumour. Our patient had a rare presentation of GIST of the jejunum, presenting with massive lower GI bleeding that necessitated pre-operative blood transfusion and urgent surgery. Despite the young age of our patient compared to the typical GIST presentation age, GIST should always be considered a differential diagnosis in patients presenting with massive GI bleeds.
Surgical resection and radiologic embolisation are the most effective methods in the management of GISTs with acute bleeding. At present, surgical resection remains the mainstay approach in treating patients with localised, nonmetastatic GIST. Radio embolisation, despite being less invasive, was not feasible in our case due to the patient’s haemodynamic instability, necessitating emergent surgical exploration.
CONCLUSION
This case highlights the diagnostic and management challenges posed by GISTs, particularly when presenting with atypical features such as massive lower GI bleeding in a relatively young patient. Despite its rarity in the jejunum, GIST should be included in the differential diagnosis of GI bleeding, especially when conventional interventions fail to stabilise the patient. Surgical resection remains the mainstay treatment for localised, non-metastatic GISTs, offering a potentially curative approach even in emergent situations. Clinicians should maintain a high index of suspicion for GISTs in patients with unexplained GI bleeding, ensuring timely diagnosis and appropriate management to optimise outcomes. Further, research is warranted to better understand the clinical spectrum and optimal therapeutic strategies for GISTs, particularly in uncommon locations such as the jejunum.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation:
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Gastric Stromal Tumors. Reappraisal of Histogenesis. Am J Surg Pathol. 1983;7:507-19.
- [CrossRef] [PubMed] [Google Scholar]
- A Sporadic Small Jejunal GIST Presenting with Acute Lower Gastrointestinal Hemorrhage: A Review of the Literature and Management Guidelines. Indian J Surg. 2015;77(Suppl 1):143-6.
- [CrossRef] [PubMed] [Google Scholar]
- Severe Upper Gastrointestinal Bleeding from Gastrointestinal Stromal Tumor of the Stomach. Rom J Morphol Embryol. 2016;57:1397-401.
- [Google Scholar]
- Surgical Treatment of Giant Gist with Acute Gastrointestinal Bleeding: Case Report. Int J Surg Case Rep. 2018;53:354-7.
- [CrossRef] [PubMed] [Google Scholar]
- Incidence of Sarcoma Histotypes and Molecular Subtypes in a Prospective Epidemiological Study with Central Pathology Review and Molecular Testing. PLoS One. 2011;6:e20294.
- [CrossRef] [PubMed] [Google Scholar]
- Massive Gastrointestinal Haemorrhage Unusual Presentation of Gastrointestinal Stromal Tumors of the Jejunum: Case Report and Literature Review. Cureus. 2021;13:e14266.
- [CrossRef] [Google Scholar]
- Global Epidemiology of Gastrointestinal Stromal Tumours (GIST): A Systematic Review of Population-based Cohort Studies. Cancer Epidemiol. 2016;40:39-46.
- [CrossRef] [PubMed] [Google Scholar]
- Gastrointestinal Stromal Tumors: A Review of Case Reports, Diagnosis, Treatment, and Future Directions. ISRN Gastroenterol. 2012;2012:595968.
- [CrossRef] [PubMed] [Google Scholar]
- Gastrointestinal Stromal Tumors: A Comprehensive Review. J Gastrointest Oncol. 2019;10:144-54.
- [CrossRef] [PubMed] [Google Scholar]
- Gain-of-Function Mutations of c-kit in Human Gastrointestinal Stromal Tumors. Science. 1998;279:577-80.
- [CrossRef] [PubMed] [Google Scholar]






