Translate this page into:
Study of Clinical and Haematological Profile of Vitamin b12 Deficiency and to Check Response to Vitamin b12 Therapy
*Corresponding author: Archana Aher, Associate Professor, Department of Medicine, Government Medical College and Hospital, Nagpur, Maharashtra, India. drarchanaaher@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Aher A, Navghare P, Zawar S. Study of clinical and haematological profile of vitamin b12 deficiency and to check response to vitamin b12 therapy. Vidarbha J Intern Med. 2023;33:73-6. doi: 10.25259/VJIM_43_2022
Abstract
Objectives:
Vitamin B 12 (cobalamin) deficiency has been a known clinical entity for long time. Average Indian diet is deficient in vitamin B12. Deficiency of vitamin is associated with a wide spectrum of haematologic and neuropsychiatric disorders that can often be reversed by early diagnosis and prompt treatment. Diagnosis is easy and treatment ids cheap. Hence, we conducted this study to find patterns of presentation in patients with vitamin B12 deficiency with haematological profile and to check response to therapy.
Material and Methods:
A longitudinal study was conducted between September 2016 and November 2018 in the Department of Medicine at Government Medical College, Nagpur, India. One hundred patients whose serum vitamin B12 level was <250 pg/mL were included in the study.
Results:
Mean vitamin B12 level in patients was 162.64 ± 43.31 pg/mL. Most of patients were male and deficiency was common among vegetarians. History of alcoholism was present in 41% of patients. The most common complaint was fatigue present in 87% of patients. Most common neuropsychiatric complaint was tingling and numbness (14%). Most common sign elicited was pallor in 96% of patients while icterus and knuckle hyperpigmentation was present in 57% and 74% of patients, respectively. Mean haemoglobin (Hb) among cases was 6.09 ± 2.21 g/dL. After treatment, all the patients showed a dramatic response in Hb level, mean corpuscular volume, red cell distribution width and mean platelet volume.
Conclusion:
Deficiency of vitamin B12 is associated with plethora of clinical manifestations. Males, alcoholics and vegetarians are at risk. Early treatment halts late complications.
Keywords
Vitamin B12
Vegetarian
Alcoholism
INTRODUCTION
Vitamin B12 deficiency, also known as cobalamin deficiency, is the medical condition of low blood levels of vitamin B12. Animal proteins, mainly meats and eggs are important sources of cobalamin.
The average Indian diet is deficient in cobalamin[1,2] and deficiency is common in India due to religious and economic reasons.[3,4] Prevalence rates of deficiency of 47–71% have been reported among adults.[5]
Vitamin B12 deficiency can cause a wide range of symptoms. These usually develop gradually but can worsen if the condition goes untreated. A mild deficiency may not cause any discernible symptoms, but as the deficiency becomes more severe and symptoms of anaemia may result, such as fatigue, light-headedness, rapid heartbeat, rapid breathing and pale colour to the skin. It may also cause easy bruising or bleeding, including bleeding gums. Gastrointestinal side effects including sore tongue, stomach upset, weight loss and diarrhoea or constipation.
If timely the deficiency is not corrected, nerve cell damage can result. If this happens, vitamin B12 deficiency may result in tingling or numbness to the fingers and toes, difficulty walking, mood changes, depression, memory loss, disorientation and, in severe cases, dementia. Vitamin B12 deficiency is also associated with raised plasma homocysteine levels, which is a risk factor for cardiovascular disease.[6]
Haematological profile of vitamin B12 deficiency varies from normal blood parameters to classical features of megaloblastic anaemia such as quite elevated mean corpuscular volume (MCV), macro-ovalocytes and hypersegmented neutrophils (six or greater lobed) on peripheral smear (PS), reduce retic count and modest increase in indirect bilirubin. Vitamin B12 deficiency can affect all haematopoietic cell lines, although red blood cells are reduced in mild-to-moderate anaemia while white blood cells and platelet count are reduced in severe cases.
Bone marrow morphology is characteristically abnormal. Marked erythroid hyperplasia is present as a response to defective red blood cell production (ineffective erythropoiesis). Megaloblastic changes in the erythroid series include abnormally large cell size and asynchronous maturation of the nucleus and cytoplasm, that is, cytoplasmic maturation continues while impaired DNA synthesis causes retarded nuclear development. In the myeloid series, giant bands and meta-myelocytes are characteristically seen. Megaloblastic anaemia must be an important differential diagnosis in patients presenting with pancytopenia.[7,8] Hence, making diagnosis of vitamin B12 deficiency is very difficult task especially in patient with mild vitamin B12 deficiency with normal haematological profiles.
Diagnosis is typically based on blood levels of vitamin B12. Elevated methylmalonic acid levels may also indicate a deficiency. Treatment consists of using vitamin B12 by mouth or by injection; initially in high daily doses, followed by less frequent lower doses as the condition improves. If a reversible cause is found, that cause should be addressed if possible. If no reversible cause is found – or when found it cannot be eliminated – lifelong vitamin B12 supplementation is usually recommended. Effective therapy reverses megaloblastosis in 24 h and reestablishes normal marrow haematopoiesis in 48 h. Rising red blood cell count and haemoglobin (Hb) will take 1 week; normalisation of the complete blood count (CBC) requires about 68 weeks.
MATERIAL AND METHODS
This was hospital-based longitudinal study conducted at Government Medical College, Nagpur from November 2016 to October 2018. Approval for the study was taken by the Institutional Ethics Committee and informed written consent was obtained from all the subjects before conducting the study. Patients who were admitted to medicine ward with Hb <13 g% in males and <12 g% in females are considered anaemic and were screened for vitamin B12 deficiency. The serum vitamin B12 level was measured by chemiluminescent immunoassay in all patients. One hundred such patients with serum vitamin B12 level <250 pg/mL were enrolled in study.
Complaints on presentation, detailed general and systemic examination and all investigations were noted on a predesigned pro forma. Each patient had undergone a battery of investigations which included CBC, peripheral smear, retic count, bone marrow (if willing), liver function test and renal function test.
Every patient was advised to take six injections of vitamin B12 1000 µg IM given at 7 days interval. The patient explained about allergic reactions such as nausea, headache, erythema, chromaturia, headache and local site reactions, though they are rare. Patients followed up after 1st, 3rd and 6th week with repeat CBC and peripheral smear. Patients were looked for clinical and haematological improvement after the treatment.
Continuous variables were presented as mean standard deviation. Categorical variables were expressed in frequency and percentages. Haematology parameters were compared at different follow-up periods by performing one-way analysis of variance test. Correlation of severity of Hb with serum vitamin B12 levels was assessed to determine Pearson’s correlation coefficient. P < 0.05 was considered as statistical significance. Statistical software STATA version 14.0 is used for data analysis.
RESULTS
Mean vitamin B12 in cases was 162.64 ± 43.31 pg/mL. Age group ranged from 13 to 67 years. Most patients belonged to age group 21 to 30, that is, 3rd decade. Males (64%) were outnumbering females with male-to-female ratio 1.78:1. Most of patients (64%) were vegetarian by diet and 41% cases were addicted to alcohol. Most of patients (87%) presented with fatigue, while nausea with poor appetite was present in 63% patients. Neuropsychiatric manifestations were present in form of tingling and numbness (14%), difficulty in walking (6%), memory loss (2%) and depression and psychosis (2%).
Most common sign elicited was pallor (93%). Skin hyperpigmentation and icterus were present in 74% and 57% patients, respectively. Organomegaly was present in form of hepatomegaly and splenomegaly in 45% and 23%, respectively. Haemic murmur was present in only three patients as shown in Figure 1.
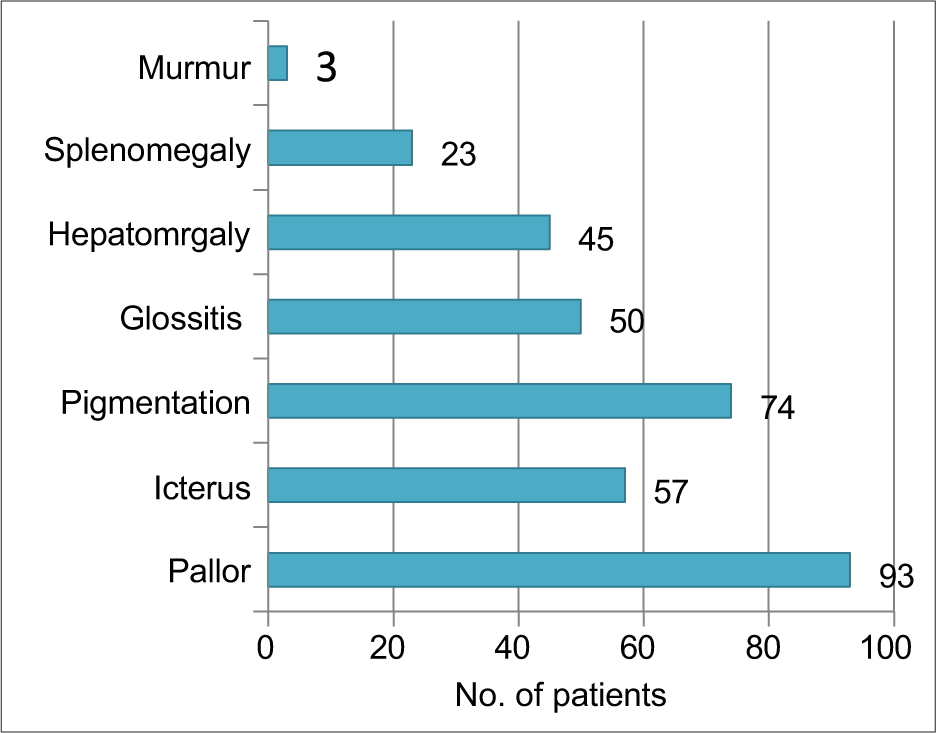
- Distribution of signs.
Among neurological signs, impaired joint and position were most common (27%). Positive Babinski sign was present in 18 patients. Absent ankle reflex and exaggerated knee reflex were present in 18% and 14% of patients, respectively. However, there were only two patients with optic atrophy.
The correlation between Hb and serum B12 levels is shown in Figure 2. Mean Hb in cases was 6.09 ± 2.21 g/dl, and maximum number of patients (66%) had severe anemia (Hb<7gm%). There was statistically significant correlation between Hb percentage and serum vitamin B 12 level with P < 0.0001 with r value + 0.4974.
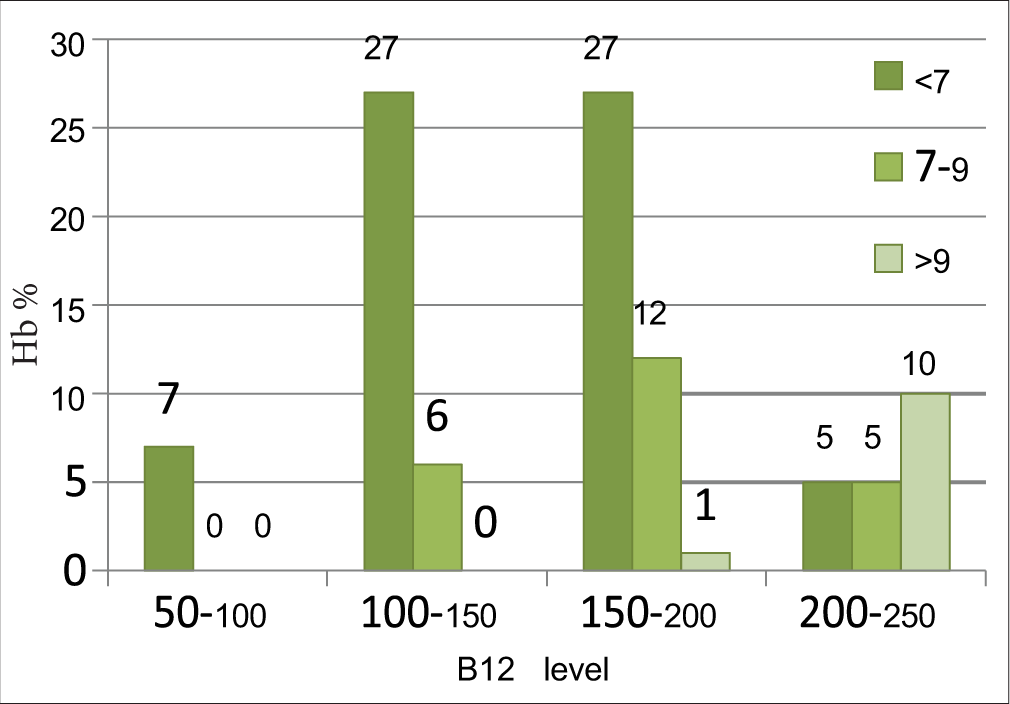
- Correlation between haemoglobin (g/dL) and serum vitamin B12 level (pg/mL).
Baseline reticulocyte count in case was 1.6 ± 0.43%. Mean total, direct and indirect bilirubin were 2.19 ± 0.63 mg/dl, 0.70 ± 0.30 mg/dL and 1.66 ± 1.71 mg/dL, respectively.
All patients showed clinical improvement after treatment. Furthermore, there was significant increase in Hb and reduction in MCV after treatment with P < 0.0001. Response to vitamin B12 could also be judged by reduction in red cell distribution width (RDW) and increase in mean platelet volume mean platelet volume (MPV) as shown in Tables 1 and 2.
| Parameter | Mean | SD | Range |
|---|---|---|---|
| Hb (g/dL) | 6.09 | 2.21 | 1.8–11.3 |
| MCV (fl) | 97.31 | 13.02 | 75.4–132.2 |
| MCH (pg) | 34.35 | 1.96 | 30.5–39.8 |
| MCHC (g/dL) | 34.07 | 1.58 | 30.5–37.3 |
| TLC (/µL) | 3911 | 2055.48 | 700–8100 |
| RBC (×106/µL) | 2.12 | 0.40 | 1.21–3.26 |
| PLT (× 105/µL) | 1.49 | 0.84 | 0.28–3.45 |
| MPV (fl) | 7.86 | 0.80 | 6–9.7 |
| RDW (%) | 16.37 | 0.97 | 14.8–19.1 |
Hb: Haemoglobin, MCV: Mean corpuscular volume, MCH: Mean corpuscular haemoglobin, MCHC: Mean corpuscular haemoglobin concentration, TLC: Total leucocyte count, RBC: Red blood cells, PLT: Platelet count, MPV: Mean platelet volume, RDW: Red cell distribution width, MPV: Mean platelet volume, SD: Standard deviation
| Parameters | Pre-treatment | Post-treatment | P-value |
|---|---|---|---|
| Hb (g/dL) | 6.09±2.21 | 8.93±1.90 | P<0.0001, |
| RBC (×106/ L) | 2.12±0.40 | 2.50±0.36 | HS |
| TLC (/µL) | 3911±2055.48 | 7220±1429.17 | |
| PLT (×105/ L) | 1.49±0.84 | 2.87±0.64 | |
| MCV (fl) | 97.31±13.02 | 83.30±6.16 | |
| RDW (%) | 16.37±0.97 | 12.14±1.00 | |
| MPV (fl) | 7.86±0.80 | 8.34±0.72 |
Hb: Haemoglobin, RBC: Red blood cells, TLC: Total leucocyte count, PLT: Platelet count, MCV: Mean corpuscular volume, RDW: Red cell PLT: Platelet count, MCV: Mean corpuscular volume, RDW: Red cell distribution width, MPV: Mean platelet volumes
Most common finding on the peripheral smear was macro ovalocytes (96%). Other findings were hypersegmented neutrophils (25%) and thrombocytopenia (53%). Furthermore, bone marrow findings were consistent with megaloblastic picture.
DISCUSSION
Incidence of vitamin B12 deficiency in India is unknown but recent studies have shown that it is more common than we thought.
In our study, mean age of patients was 34.17 ± 12.32 years with most of patients (36%) from age group 21–30 years, that is, 3rd decade but all age groups were affected. The peak incidence in another Indian study done by Khanduri et al.,[9] was seen in the age group of 10–30 years. In Caucasian and Chinese populations, megaloblastic anaemia is reported to occur in the older age groups. As in India, it affects all age groups and is possibly related to an inadequate diet due to religious and financial issues. Males were commonly affected than females, which was similar to studies conducted by Paudel et al.[10] and Bhatia et al.[11]
Majority of patients were vegetarian in diet. Bhole et al.[12] and Suthar and Shah,[13] also in their study, also observed that majority of cases were vegetarian. Because vitamin B12 does not occur in vegetarian foods, strict vegetarian is at high risk of developing deficiency. Furthermore, consumption of alcohol is known to cause vitamin B12 deficiency, so result of our study is comparable with the previous studies.[14]
Most of patients were presented with constitutional symptoms such as fatigue, light-headedness and dizziness, that is, symptoms secondary to anaemia and also most common finding was pallor. These symptoms and findings were comparable with various other Indian studies.[15,16] Among patient with neurological involvement, most common finding was impaired joint and position sense. Suthar and Shah[13] also found that 40% patients with neurological feature were having impaired joint position. Neurological manifestation in vitamin B12 deficiency is attributed to selective vulnerability of posterior and lateral column to demyelination.
Most common finding on peripheral smear examination was macrocytosis and also most of bone marrow examination shows megaloblastic picture which is consistent previous studies.[11,17] Mean bilirubin was raised and was attributed to ineffective erythropoiesis leading increased haemolysis.
Mean Hb and MCV were 6.09 ± 2.21 g/dL and 97.31 ± 13.02 fl. About 41% of patients were having MCV >100 fl and 66% were having Hb <7g/dL. However, mean corpuscular Hb and mean corpuscular Hb concentration were within normal limits. Mean Hb and MCV of study population by Srikanth[18] was 6.195 ± 2.54 g/dL and 98.47 ± 10.14 fl. Hence, most of patients were having severe anaemia and increased MCV in patients was attributed to macrocytosis due to vitamin B12 deficiency.
All patients showed clinical improvement and also haematological improvement in form increase in Hb. Hence, it is advisable in patients with vitamin B12 deficiency not responding to treatment to search for other causes and to check compliance. Reduction in MCV, reduction in RDW and increase in MPV can also use as alternative to check response vitamin B12 therapy. Our results were comparable with study done on Turkish population by Aktas et al.[19]
CONCLUSION
Vitamin B12 deficiency is not uncommon in India. Vegetarians and alcoholics are at risk. Deficiency presented with plethora of clinical manifestations. Diagnosis is easy and treatment is easy and affordable. Early diagnosis and treatment avoid late consequences at primary level itself and provide cost-effective management in resource-limited countries like India. MPV and RDW can be used to check response to vitamin B12 therapy. Large prospective studies are needed for confirmation of our findings.
Ethical approval
The research/study had been approved by the Institutional Ethics Committee, approval number 967, dated 4/1/2017.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Vegetarianism and Vitamin B-12 (cobalamin) deficiency. Am J Clin Nutr. 2003;78:3-6.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence of cobalamin (Vitamin B-12) and folate deficiency in India--audi alteram partem. Am J Clin Nutr. 2001;74:157-9.
- [CrossRef] [PubMed] [Google Scholar]
- Staging Vitamin-B12 (cobalamin) status in vegetarians. Am J Clin Nutr. 1994;59:1213S-22.
- [CrossRef] [PubMed] [Google Scholar]
- Hyperhomocysteinemia and elevated methylmalonic acid indicate a high prevalence of cobalamin deficiency in Asian Indians. Am J Clin Nutr. 2001;74:233-41.
- [CrossRef] [PubMed] [Google Scholar]
- Vitamin B12 deficiency and hyperhomocysteinemia in rural and urban Indians. J Assoc Physicians India. 2006;54:775-82.
- [Google Scholar]
- Conclusions of a WHO Technical Consultation on folate and Vitamin B12 deficiencies. Food Nutr Bull. 2008;29:S238-44.
- [CrossRef] [PubMed] [Google Scholar]
- Pancytopenia in nutritional megaloblastic anaemia. A study from north-west India. Trop Geogr Med. 1989;41:331-6.
- [Google Scholar]
- Occult cobalamin and folate deficiency in Indians. Natl Med J India. 2005;18:182-3.
- [Google Scholar]
- Study of Vitamin B12 status and the consequential clinico-hematological profile in healthy vegetarian population in Nepal. J Chitwan Med Coll. 2017;7:41-6.
- [CrossRef] [Google Scholar]
- Vitamin B12 deficiency in India: Mean corpuscular volume is an unreliable screening parameter. Natl Med J India. 2012;25:336-8.
- [Google Scholar]
- A study of correlation between Vitamin B12 deficiency and its clinical, haematological and electrophysiological Parameters. Int J Med Sci Educ. 2014;1:187-94.
- [Google Scholar]
- Evaluation of clinical profile and hematological parameters of cases of megaloblastic anemia. Natl J Community Med. 2017;8:592-6.
- [Google Scholar]
- Clinical profile and response in patients with megaloblastic anemia. Int J Med Sci Public Health. 2016;5:304-6.
- [CrossRef] [Google Scholar]
- Vitamin B12-homocysteine interaction and the efficacy of B12 therapy in relation to anemia and neurological disease in a North Indian population. Int J Nutr Pharmacol Neurol Dis. 2012;2:61-9.
- [CrossRef] [Google Scholar]
- Clinico-haematological and biochemical evaluation of macrocytic anaemia: A prospective cross sectional study. Int J Curr Adv Res. 2017;6:5553-6.
- [Google Scholar]
- A study of the prevalence of serum Vitamin B12 and folic Acid deficiency in eWestern Maharashtra. J Family Med Prim Care. 2015;4:64-8.
- [CrossRef] [PubMed] [Google Scholar]
- Megaloblastic anemia-A clinical spectrum and a hematological profile: The day-to-day public health problem. Med J D Y Patil Univ. 2016;9:307-10.
- [CrossRef] [Google Scholar]
- Effects of Vitamin B12 treatment on hematological parameters. Acta Med Anatol. 2014;2:6-8.
- [CrossRef] [Google Scholar]






