Translate this page into:
Study of Clinical Profile and Treatment Pattern of Scrub Typhus Patients in Tertiary Care Hospital
*Corresponding author: Shubham Ingle, Junior Resident, Department of Medicine, Government Medical College and Hospital, Nagpur, Maharashtra, India. shubhamingle131@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Ingle S, Rajkondwar A. Study of clinical profile and treatment pattern of scrub typhus patients in tertiary care hospital. Vidarbha J Intern Med 2022;32:10-4.
Abstract
Objectives:
To assess the clinical features, complications and treatment of scrub typhus patients in tertiary care hospital.
Material and Methods:
A total of 100 patients with scrub typhus, aged >12 years admitted in medicine wards and intensive care units of Government Medical College, Nagpur, were included in the study. A detailed history was taken; clinical examination, all relevant investigations, and IgM ELISA test were done for the diagnosis of scrub typhus.
Results:
The most common presenting symptoms were high-grade fever with chills (80%). The most common complication was lung involvement (35%), liver damage (28%), and acute kidney injury (27%) with six cases requiring renal replacement therapy. Multisystem organ dysfunction syndrome was seen in 19% of cases. About 27% of patients had acute respiratory distress syndrome of which 16 cases needed mechanical ventilation. Of 100 patients included, 82 (82%) cases survived and were discharged, while 18 (18%) patients succumbed during hospital admission. Among the risk factor studied, systemic hypertension was associated with an increase in mortality (P = 0.014). There was a statistically significant increase in mortality (P = 0.006) in patients having electrocardiogram abnormalities in the forms of low-voltage complexes and tachycardia. Early diagnosis and rapid treatment with doxycycline (93.90%) and ceftriaxone (87.80%) were found to be associated with more survival rate.
Conclusion:
Scrub typhus is a significant disease in this part of the country; therefore, it should be kept in mind as a possible diagnosis in undifferentiated fevers even though an eschar is not found. An early diagnosis and timely antibiotic therapy may prevent further complications.
Keywords
Scrub typhus
ELISA test
Mortality
EEG
Tachycardia
Doxycycline
Ceftriaxone
INTRODUCTION
Scrub typhus is a re-emerging zoonotic disease caused by the bacterium Orientia tsutsugamushi and has become endemic in many parts of India.[1] Scrub typhus is characterised by focal or disseminated vasculitis and perivasculitis which may involve the lungs, heart, liver, spleen, and the central nervous system (CNS).[2,3] Clinical manifestations involve almost every organ and can range in severity from a mild, self-limiting disease to, if untreated, a fatal illness. Clinical manifestations include fever, headache, skin rash, lymphadenopathy, and gastrointestinal (GI) symptoms. Black eschar is a characteristic skin lesion at the site of mite bite. The complications that can be seen in patients with scrub typhus are jaundice, pneumonitis, acute respiratory distress syndrome (ARDS), septic shock, renal failure, myocarditis, meningoencephalitis and multiorgan dysfunction syndrome (MODS) may be the presenting manifestation after the 1st week of illness.[4]
However, scrub typhus is a disease that often goes undiagnosed due to its vague clinical symptoms and lack of a definitive protocol for its diagnosis. It requires laboratory confirmation for diagnosis. It should be considered as a differential diagnosis in patients with acute febrile illness including those with thrombocytopaenia, renal impairment, liver function abnormality, altered sensorium, pneumonitis, and ARDS.[5,6] A thorough search for eschar, particularly in hidden areas, is helpful for diagnosis. The main stay in scrub typhus diagnosis remains serology. The oldest test is the Weil-Felix OX-K agglutination reaction which is inexpensive, easy to perform and results are available overnight.[7]
ELISA for the detection of IgM antibodies against O. tsutsugamushi offers the advantages of being able to test a large number of samples at a time and can be automated.[8] Indirect fluorescent antibody assay is the gold standard assay for the serological detection of antibodies in scrub typhus.[9] Diagnosis and surveillance of scrub typhus are challenging, particularly in the absence of advanced diagnostic techniques. The availability and cost of other serological methods are a major problem in India.[7]
MATERIAL AND METHODS
After obtaining the Institutional Ethics Committee approval and written informed consent from patients/caregivers, this retrospective and prospective study was conducted in the department of general medicine and intensive care units (ICU) from tertiary care hospital in Central India during a period of 30 months from May 2018 to October 2020. A total of 100 patients with scrub typhus, aged >12 years admitted in medicine wards and ICU of tertiary care centre, were included in the study. Patients of age <12 years were excluded from the study.
A brief clinical history including baseline personal characteristics (chief complaints, past and personal history, physical examination, temperature, pulse, respiratory rate, blood pressure, JVP, oedema feet, cyanosis, pallor, and body mass index) was taken. All the subjects were interviewed with the pre-designed questionnaire for a detailed history and were followed by clinical examination. All relevant blood investigations (RBS, CBC, FBS, PLBS, HBA1C, and serial arterial blood gas analysis) and radiological investigations (X-ray chest PA view and electrocardiogram [ECG]) were done for all the patients. The ELISA test was done using the ‘Scrub typhus Detect TM IGM’ and this ELISA system was manufactured by InBios International, Inc., USA. Diagnosis of scrub typhus was confirmed if patients having positive scrub typhus IgM or IgG ELISA test.
Statistical analysis
Continuous variables were presented as mean ± standard deviation. Categorical variables were expressed in frequency and percentages. Categorical variables were compared by performing Chi-square test. Various obtained data were compared with mortality and survivors by performing an independent t-test. P < 0.05 was considered as statistical significance. Statistical software STATA version 14.0 was used for data analysis.
RESULTS
Of 100 hospitalised scrub typhus positive patients, 82 (82%) cases survived and were discharged, while 18 (18%) were included in the non-survival group. The mean age of the survivors was 41.59 ± 17.64 years whereas the mean age for non-survivors was 46.83 ± 16.74 years (P = 0.2529). Age (P = 0.754) and gender (0.391) distribution showed no statistically significant correlation with affection and outcome of the disease, as shown in [Table 1].
| Demographic data | Outcome (%) | Total (%) | |
|---|---|---|---|
| Survivors (n=82) | Non-survivors (n=18) | ||
| Age group (years) | |||
| ≤20 | 10 (12.20) | 1 (5.56) | 11 (11) |
| 21–30 | 16 (19.51) | 3 (16.67) | 19 (19) |
| 31–40 | 16 (19.51) | 3 (16.67) | 19 (19) |
| 41–50 | 13 (15.85) | 2 (11.11) | 15 (15) |
| 51–60 | 14 (17.07) | 6 (33.33) | 20 (20) |
| 61–70 | 11 (13.41) | 2 (11.11) | 13 (13) |
| ≥71 | 2 (2.44) | 1 (5.56) | 3 (3.0) |
| Sex | |||
| Male | 32 (39.02) | 9 (50) | 41 (41) |
| Female | 50 (60.98) | 9 (50) | 59 (59) |
Patients presented with diverse clinical symptoms and signs. The most common presenting symptoms were high-grade fever with chills, headache, cough, shortness of breath, and altered sensorium, as depicted in [Figure 1].
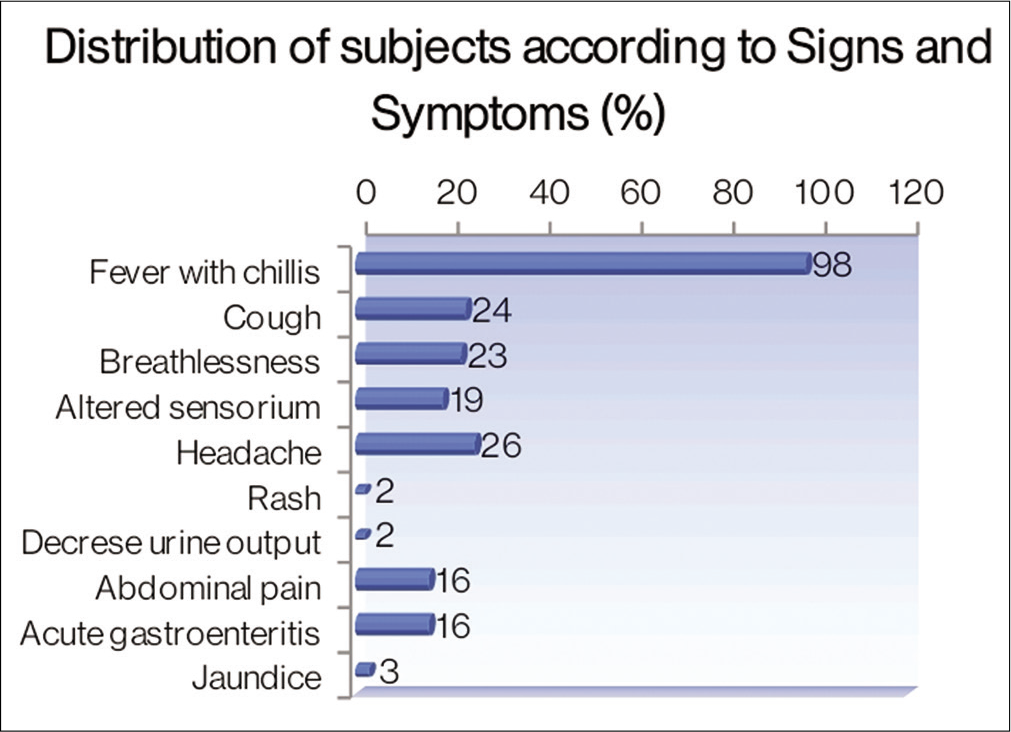
- Distribution of signs and symptoms by outcome.
The majority (37%) of cases show respiratory system involvement followed by liver (35%), renal (28%), thrombocytopaenia (26%), involvement of cutaneous system presented as eschar (22%) and CNS (13%) involvement in the form of meningitis and encephalitis. Out of 100 cases, 27% of patients had associated comorbidities, of which 19% of cases had type 2 diabetes mellitus while 8% had systemic hypertension. There was a statistically significant correlation observed that is, P < 0.05 in between respiratory system damage, acute kidney injury, and patient outcome as well as a significant association found between systemic hypertension and mortality, as shown in [Table 2].
| Parameters | Outcome (%) | P-value | |
|---|---|---|---|
| Survivors (n=82) | Non-survivors (n=18) | ||
| Organ system | |||
| Eschar | 17 (20.73) | 5 (27.78) | 0.513 |
| CNS | 10 (12.20) | 3 (16.67) | 0.623 |
| RS | 21 (25.61) | 16 (88.89) | 0.001 (S) |
| Liver | 26 (31.71) | 9 (50) | 0.151 |
| Renal | 19 (23.17) | 9 (50) | 0.022 (S) |
| Thrombocytopaenia | 24 (29.27) | 2 (11.11) | 0.112 |
| Associated comorbidities | |||
| Diabetes mellitus | 13 (15.85) | 6 (33.33) | 0.087 |
| Hypertension | 4 (4.88) | 4 (22.22) | 0.014 (S) |
On chest X-ray, 35% of patients had lung involvement in terms of bilateral basal (23%) and diffuse lung infiltrates (12%). An increase in mortality was observed in patients having bilateral basal lung infiltrates as compared to those with bilateral diffuse lung infiltrates and normal lung. Thus, there was a statistically significant correlation found between patients’ outcomes and lung involvement based on the X-ray chest (P = 0.001). 30% of the patients had ECG abnormalities - of these 22% has had sinus tachycardia, and 8% had low voltage complexes on ECG. Patients with ECG changes had statistically significant increase in mortality [Figure 2].
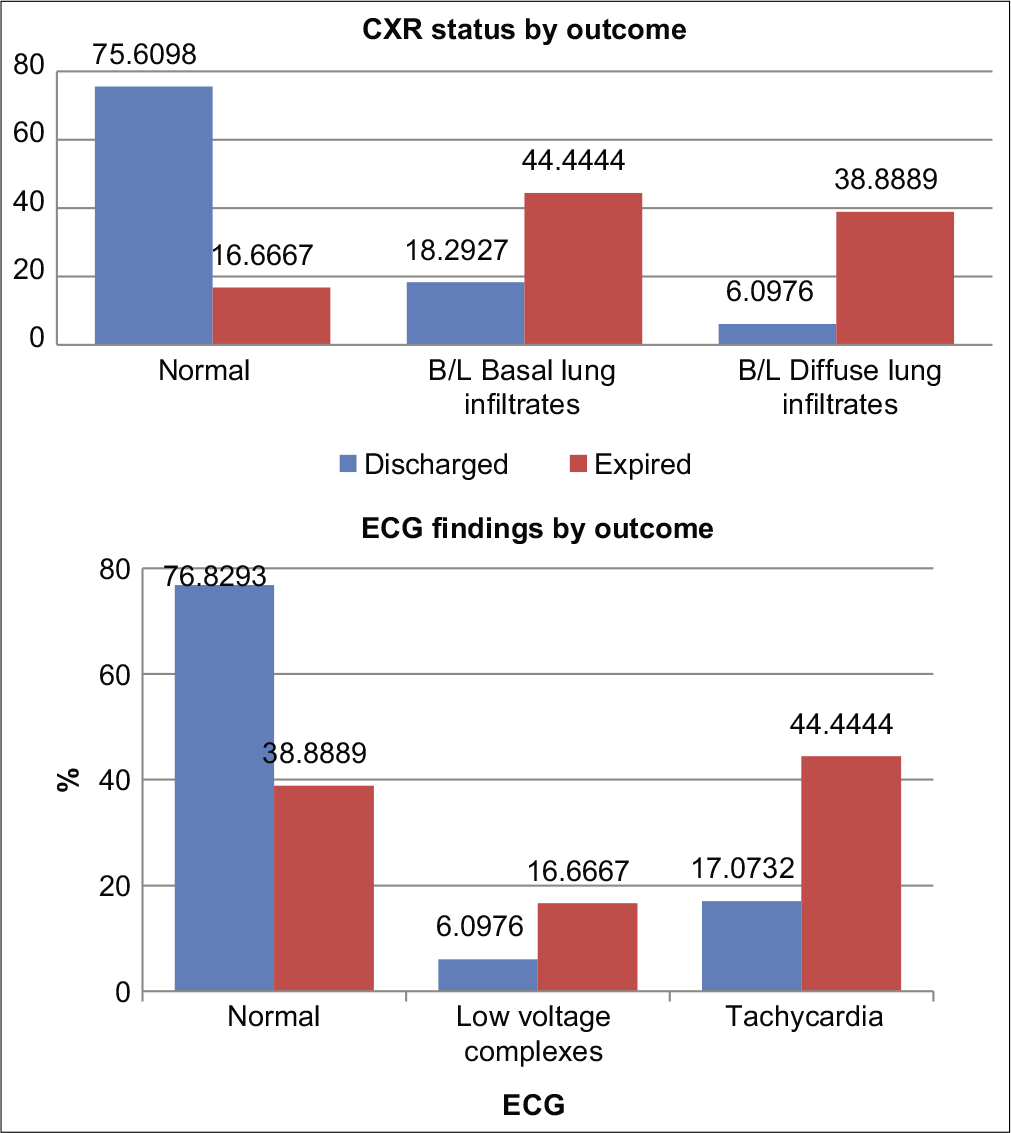
- Correlation between radiological findings and patient outcome.
There was increase in mortality associated with decrease in arterial PO2 level <71.66 mmHg (P = 0.001) and decrease in arterial PO2/FIO2 ratio<300 (P = 0.001). Blood urea level >100.88 mg/dl and blood creatinine>3.03 mg/dl were associated with a higher mortality rate than other laboratory parameters (P ≤ 0.0001). Furthermore, AST>229.72 IU/dl and ALT>193.5 IU/dl as well as serum total bilirubin level>3.24 mg/dl were found to be associated with an increase in mortality rate, P = 0.0263, 0.0061, and 0.0003, respectively [Table 3].
| Variable | Outcome | P-value | |
|---|---|---|---|
| Survivors (n=82) | Non-survivors (n=18) | ||
| ABG analysis | |||
| Duration of stay | 7.51±5.33 | 5±2.11 | 0.053 |
| PO2 | 91.34±15.66 | 71.66±21.02 | 0.0001 (S) |
| PCO2 | 42.57±9.22 | 47.98±19.14 | 0.0758 |
| pH | 7.36±0.07 | 7.24±0.08 | 0.0001 (S) |
| PO2FIO2 | 365.68±92.3 | 198.8±120.56 | 0.0001 (S) |
| Laboratory parameters | |||
| Urea | 47.41±39.08 | 100.88±77.18 | 0.0001 (S) |
| Creatinine | 1.29±0.69 | 3.03±2.37 | 0.0001 (S) |
| ALP | 102.29±144.08 | 150.78±139.96 | 0.1969 |
| AST | 120.28±172.28 | 229.72±242.55 | 0.0263 (S) |
| ALT | 94.39±110.45 | 193.5±219.88 | 0.0061 (S) |
| Total Bilirubin | 1.66±1.24 | 3.24±2.75 | 0.0003 (S) |
ABG: Arterial blood gas, ALP: Alkaline phosphatase, ALT: Alanine transaminase, AST: Aspartate transaminase
Of the 100 cases, 25% of cases had ARDS (2% mild, 16% moderate, and 7% severe), 27% had acute kidney injury and 16% of patients developed respiratory failure requiring endotracheal intubation and mechanical ventilator while 6% needed renal replacement therapy. Patients required ventilator support and RRT had decreased survival rate compare to other patients (P = 0.0001) [Table 4], so which was associated with a poor prognosis.
| Variables | Outcome (%) | P-value | |
|---|---|---|---|
| Survivors (n=82) | Non-survivors (n=18) | ||
| Therapy | |||
| Ventilatory support | 4 (4.88) | 12 (66.67) | 0.0001 (S) |
| RRT | 1 (1.22) | 5 (27.78) | 0.0001 (S) |
| Treatment | |||
| Ceftriaxone | 72 (87.80) | 10 (55.56) | 0.001 (S) |
| Doxycycline | 77 (93.90) | 18 (100) | 0.282 |
| Piperacillin, tazobactam | 7 (8.54) | 7 (38.89) | 0.001 (S) |
| Azithromycin | 37 (45.12) | 5 (27.78) | 0.177 |
| Meropenem | 0 (0.00) | 1 (5.56) | 0.032 (S) |
| Rifampicin | 5 (6.10) | 0 (0.00) | 0.282 |
RRT: Renal replacement therapy
Out of 100 patients, 95 patients received inj. doxycycline, while 82 patients received inj. ceftriaxone. Seventy-seven patients out of 95 patients receiving doxycycline survived while doxycycline had no significant role in 18 non-survived patients. The use of doxycycline showed no statistically significant decrease in mortality rate but for ceftriaxone, recovery was significantly more and showed statistically significant effects on patient outcome, as shown in [Table 4].
DISCUSSION
The diagnostic modality used to diagnose scrub typhus in our patients was IgM ELISA immunofluorescence test which has a sensitivity of 90–95%. The tested polymerase chain reaction protocol has a specificity of 100%. This febrile illness affects previously healthy active persons and if undiagnosed or diagnosed late may prove life threatening. Most of the cases in the present study presented with non-specific symptoms pertaining to respiratory or GI tract such as the usual viral illnesses, the duration of fever in the majority of cases was usually around 7 days. In addition to headache, high-grade fever with chills, nausea, vomiting, cough, and breathlessness was seen in a significant number of cases. Nearly one-fifth of our patients presented with altered sensorium. These findings are similar to a study done by Verghese et al.[10] and Vivekanandan et al.[11]
Eschar is a black necrotic lesion resembling a cigarette burn usually found in areas where the skin is thin, moist, or wrinkled and where the clothing is tight. In the present study, the pathognomonic eschar was present in 22% of patients, a figure considerably higher than a previous study from the Himalayan region (9.5% of patients), but lesser than that reported from South India (43.5%)[13] and Jeju Island in South Korea (75.8%).[12,14] This variation in the presence of eschar may be explained by the geographic distribution of different strains of the organism. Eschar may be associated with regional or generalised lymphadenopathy, but this was not found in our study. A maculopapular rash, as a presenting feature, was seen in 2% of our patients which is correlated with the previous studies.[10,15] The GI manifestations such as abdominal pain (11%), vomiting (13%), and diarrhoea occurred in 13% of patients. This highlights the fact that febrile patients of scrub typhus can also present with prominent GI symptoms.
Among the laboratory findings, thrombocytopaenia was noted in 26% of cases which is correlated with the other Indian study done by Krishnamurthy et al.[16] However, among the laboratory parameters, the most consistent abnormality noticed was the elevation of liver enzymes which were present in 28% of the cases. Acute kidney injury was the second most common laboratory abnormality found in 27% of patients. Similar abnormalities have been observed in other studies also.[17] Thirty-five patients had lung infiltrates on the X-ray chest of the 23 patients who had bilateral basal infiltrates, while 12 had bilateral diffuse lung involvement. About 25% of the study population had evidence of ARDS whereas earlier studies have reported ARDS in 8–34%.[13,17,18] Two patients had mild; 16 cases had moderate while seven cases had severe ARDS. Eighteen cases from the study required ventilatory support. MODS was seen in 19% of cases which is correlated with the previous study.[18] Moreover, MODS was the leading cause of death in the present study.
Acute kidney injury was noted in 27% of patients of which 6% needed RRT which is lower than the other study reporting 53% of cases with renal failure.[19] In the present study, the case fatality rate was 18%. Data from other Indian studies have shown that the case fatality rate in scrub typhus has ranged from 1.2% to as high as 46.3% depending on the complications.[17,20,21] Deaths are attributable to late presentation, delayed diagnosis, and drug resistance.[2]
Thorough knowledge of the clinical features of scrub typhus including its various complications and its varied presentations is especially important to provide early empiric therapy in appropriate cases which may be lifesaving. Doxycycline 200 mg/day is the treatment of choice for scrub typhus. Other antibiotics useful for the treatment of this infection are chloramphenicol, azithromycin, and rifampicin. Rapid resolution of fever following doxycycline is so characteristic that it can be used as a therapeutic test.[2] Nearly 80% of our cases responded to doxycycline and other standard of care. Patients who received Ceftrioxane had a significantly higher survival. However, this needs to be verified with further studies comparing it with the severity of sickness on presentation. The strength of this study is that it is the largest prospective and retrospective study that has been carried out in Central India.
CONCLUSION
Scrub typhus is an emerging infectious disease presenting as an acute febrile illness with non-specific signs and symptoms. High-grade fever lasting for more than 7 days was the most common presenting complaint in the present study. However, it is a significant disease in this part of the country; therefore, it should be kept in mind as a possible diagnosis in undifferentiated fevers even if an eschar is not found. Early diagnosis and rapid treatment with empirical medication include doxycycline and ceftriaxone for optimal outcomes. If not treated in time, the patient can go in for organ failure leading to severe morbidity and high mortality. Thus, early recognition and appropriate intervention are the key factors for a favourable outcome.
Declaration of patient consent
Consent of Patient/Legally authorised Representative have been taken.
Financial support and sponsorship
Nil.
Conlicts of interest
There are no conflicts of interest.
References
- Scrub typhus in a tertiary care hospital in North India. Am J Trop Med Hyg. 2016;95:447-51.
- [CrossRef] [PubMed] [Google Scholar]
- Scrub typhus involving central nervous system, India, 2004-2006. Emerg Infect Dis. 2010;16:1641-3.
- [CrossRef] [PubMed] [Google Scholar]
- Scrub typhus complicated by acute respiratory distress syndrome and multiorgan failure; an unrecognized alarming entity in central India: A report of two cases. J Family Med Prim Care. 2014;3:80-3.
- [CrossRef] [PubMed] [Google Scholar]
- Scrub typhus with multi-organ dysfunction syndrome and immune thrombocytopenia: A case report and review of the literature. J Med Case Rep. 2019;13:358.
- [CrossRef] [PubMed] [Google Scholar]
- Scrub typhus in patients reporting with acute febrile illness at a tertiary health care institution in Goa. Indian J Med Res. 2012;136:1020-4.
- [Google Scholar]
- Relevance of Weil Felix in the diagnosis of scrub typhus in India. J Assoc Physicians India. 2006;54:619-21.
- [Google Scholar]
- Principle and Practices of Clinical Microbiology New Jersey, United States: John Wiley & Sons; 2006.
- [Google Scholar]
- Rickettsiosis and the international traveler. Clin Infect Dis. 2004;39:1493-9.
- [CrossRef] [PubMed] [Google Scholar]
- Scrub typhus in South India: Clinical and laboratory manifestations, genetic variability, and outcome. Int J Infect Dis. 2013;17:e981-7.
- [CrossRef] [PubMed] [Google Scholar]
- Profile of organ dysfunction and predictors of mortality in severe scrub typhus infection requiring intensive care admission. Indian J Crit Care Med. 2014;18:497-502.
- [CrossRef] [PubMed] [Google Scholar]
- Unusual genotypic distribution of Orientia tsutsugamushi strains causing human infections on Jeju Island. Am J Trop Med Hyg. 2014;90:507-10.
- [CrossRef] [PubMed] [Google Scholar]
- A community-based case-control study of behavioral factors associated with scrub typhus during the autumn epidemic season in South Korea. Am J Trop Med Hyg. 2009;80:442-6.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical profile of acute kidney injury in a pediatric intensive care unit from Southern India: A prospective observational study. Indian J Crit Care Med. 2013;17:207-13.
- [CrossRef] [PubMed] [Google Scholar]
- Outbreak of scrub typhus in southern India during the cooler months. Ann N Y Acad Sci. 2003;990:359-64.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical profile and improving mortality trend of scrub typhus in South India. Int J Infect Dis. 2014;23:39-43.
- [CrossRef] [PubMed] [Google Scholar]
- Scrub typhus is an under-recognized cause of acute febrile illness with acute kidney injury in India. PLoS Negl Trop Dis. 2014;8:e2605.
- [CrossRef] [PubMed] [Google Scholar]
- Scrub typhus in the northern provinces of Vietnam: An observational study of admissions to a national referral hospital. Trans R Soc Trop Med Hyg. 2014;108:739-40.
- [CrossRef] [PubMed] [Google Scholar]
- Scrub typhus in children at a tertiary hospital in Southern India: Clinical profile and complications. J Infect Public Health. 2012;5:82-8.
- [CrossRef] [PubMed] [Google Scholar]






